What Type Of Bond Holds Nitrogen Bases Together
Juapaving
Mar 14, 2025 · 6 min read
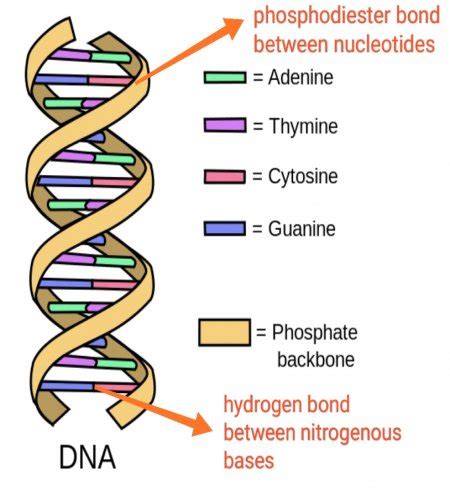
Table of Contents
What Type of Bond Holds Nitrogenous Bases Together? A Deep Dive into DNA Structure
The structure of DNA, the blueprint of life, is famously described as a double helix. This elegant, twisted ladder-like structure is held together by a complex interplay of forces, but the crucial connections between the "rungs" of the ladder – the nitrogenous bases – are formed by a specific type of bond: hydrogen bonds. Understanding the nature of these bonds is crucial to understanding how DNA replicates, transcribes its genetic code, and ultimately, dictates the characteristics of living organisms.
Hydrogen Bonds: The Glue of the Genetic Code
Hydrogen bonds are a special type of dipole-dipole attraction that occurs between molecules containing a hydrogen atom bonded to a highly electronegative atom, such as oxygen (O) or nitrogen (N). The electronegativity difference creates a significant polarity within the molecule, resulting in a partial positive charge (δ+) on the hydrogen atom and a partial negative charge (δ-) on the electronegative atom. This polarity is what allows the hydrogen bond to form.
In the context of DNA, the hydrogen bonds are formed between the nitrogenous bases adenine (A), guanine (G), cytosine (C), and thymine (T) (or uracil (U) in RNA). These bases pair up specifically: A always pairs with T (or U in RNA), and G always pairs with C. This specific pairing, dictated by the number and arrangement of hydrogen bonds, is crucial for the accurate replication and transcription of the genetic code.
The Specific Pairing: A Detailed Look
Let's examine the hydrogen bonds in each base pair more closely:
1. Adenine (A) and Thymine (T) (or Uracil (U) in RNA):
- Two hydrogen bonds connect A and T (or U). One hydrogen bond forms between the amino group (-NH2) of adenine and the carbonyl group (=O) of thymine (or uracil). The second hydrogen bond is between the N1 nitrogen of adenine and the N3 nitrogen of thymine or uracil. These bonds are relatively weaker than the G-C base pair.
2. Guanine (G) and Cytosine (C):
- Three hydrogen bonds connect G and C. The presence of three hydrogen bonds makes the G-C base pair stronger and more stable than the A-T (or A-U) base pair. These bonds involve the amino group (-NH2) of cytosine, the carbonyl group (=O) of guanine, and a nitrogen atom on both bases. The exact arrangement and positions of hydrogen bonding are critical for the fidelity of base pairing.
The number of hydrogen bonds directly influences the strength of the base pairing. The higher the number of hydrogen bonds, the stronger the interaction. This explains why G-C base pairs are more stable than A-T base pairs. This difference in stability has implications for various aspects of DNA function, such as DNA melting temperature and the stability of the DNA double helix.
Beyond Hydrogen Bonds: Other Forces at Play
While hydrogen bonds are the primary force holding the nitrogenous bases together, other intermolecular forces also contribute to the overall stability of the DNA double helix:
-
Hydrophobic interactions: The nitrogenous bases are relatively hydrophobic (water-repelling), and tend to stack on top of each other within the DNA double helix, minimizing their contact with the surrounding water molecules. This stacking interaction, known as base stacking, contributes significantly to the overall stability of the DNA double helix.
-
Van der Waals forces: These are weak, short-range forces that arise from temporary fluctuations in electron distribution around molecules. While individually weak, the cumulative effect of Van der Waals forces between the stacked bases contributes to the stability of the DNA structure.
-
Electrostatic interactions: The negatively charged phosphate backbone of the DNA molecule repels the negatively charged phosphate groups of another molecule. This repulsion prevents the two strands from collapsing completely.
The interplay of these different forces works in synergy to maintain the integrity and stability of the DNA double helix. The hydrogen bonds, while individually relatively weak, are numerous and, working together with the other forces, maintain the structure's stability. The balance between these various interactions is crucial for processes such as DNA replication, transcription, and repair.
The Importance of Base Pairing Specificity
The precise pairing of A with T (or U) and G with C is fundamental to the fidelity of DNA replication and transcription. The specific hydrogen bonding patterns allow for accurate copying of the genetic information. During replication, the DNA double helix unwinds, and each strand serves as a template for the synthesis of a new complementary strand. The specific base pairing ensures that the newly synthesized strand is an exact copy of the original strand.
Errors in base pairing, such as a mismatch between A and G, or C and T, can lead to mutations. These mutations can have significant consequences, ranging from minor phenotypic changes to severe genetic disorders. Cells possess sophisticated mechanisms to proofread and correct errors in base pairing during replication to minimize the occurrence of mutations.
Applications and Further Research
Understanding the nature of the bonds holding nitrogenous bases together has far-reaching implications in various fields. This knowledge is crucial in:
-
Drug design: Many drugs target DNA, often by interacting with the nitrogenous bases or interfering with the hydrogen bonds that hold them together. Understanding these interactions is crucial for developing effective and safe drugs.
-
Genetic engineering: Techniques like PCR (polymerase chain reaction) rely on the principles of base pairing to amplify specific DNA sequences. Understanding the strength and specificity of the hydrogen bonds is crucial to optimizing these techniques.
-
Forensic science: DNA profiling uses the principles of base pairing for identifying individuals based on their unique DNA sequences.
-
Evolutionary biology: Studying the base pairing patterns in different organisms can shed light on their evolutionary relationships and adaptations.
Ongoing research continues to explore the intricacies of base pairing, including:
-
The role of water molecules: Water molecules play a significant role in stabilizing the hydrogen bonds between bases and influencing the overall structure of DNA.
-
The effects of various environmental factors: Factors such as temperature, pH, and ionic strength can affect the stability of the hydrogen bonds and influence DNA structure and function.
-
The development of novel DNA-based technologies: Advances in our understanding of base pairing are crucial for the development of new technologies based on DNA, such as DNA computing and DNA nanotechnology.
In conclusion, the hydrogen bond is the cornerstone of DNA structure and function. Its specific nature, along with the contributions of other intermolecular forces, provides the specificity and stability required for the faithful replication and transmission of genetic information. Further research into this fascinating area continues to unravel the secrets of life itself.
Latest Posts
Latest Posts
-
What Is The Horizontal Row In The Periodic Table
Mar 14, 2025
-
What Mirror Provides The Widest Field Of View
Mar 14, 2025
-
Does Hand Sanitizer Kill Pinworm Eggs
Mar 14, 2025
-
Where Is The Magnetic Field The Strongest On A Magnet
Mar 14, 2025
-
What Is Prime Factorization Of 38
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Holds Nitrogen Bases Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
