What Is The Horizontal Row In The Periodic Table
Juapaving
Mar 14, 2025 · 6 min read
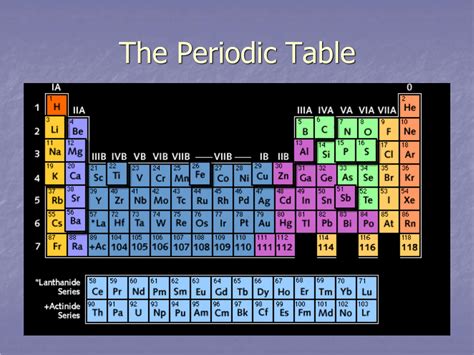
Table of Contents
What is a Horizontal Row in the Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes chemical elements in a structured grid based on their atomic number and recurring chemical properties. While the vertical columns are known as groups or families, sharing similar chemical behaviors, the horizontal rows are called periods. Understanding periods is crucial for grasping the underlying principles governing the periodic table's organization and predicting element properties. This comprehensive article will delve into the intricacies of periods, exploring their structure, trends, and the significance they hold in understanding the vast world of chemistry.
Understanding Periods: A Deeper Dive
A period in the periodic table represents a horizontal row of elements. Each period signifies a principal energy level (shell) for electrons. Elements within the same period have the same number of electron shells. As you move across a period from left to right, the number of electrons in the outermost shell, the valence shell, increases. This increase in valence electrons directly influences the element's chemical properties.
The Significance of Electron Shells
The arrangement of electrons in shells governs an atom's reactivity. Elements in the same period have electrons filling the same principal energy levels. The first period, for example, contains only hydrogen and helium, both having electrons in the first energy level (n=1). The second period, however, accommodates elements with electrons filling the second energy level (n=2), and so on.
Period Length and Electron Configuration
The length of each period is determined by the number of electrons that can occupy the subshells within a given principal energy level. The first period, with only two elements, reflects the fact that the first energy level (n=1) has only one subshell (1s), which can hold a maximum of two electrons. The subsequent periods get longer as more subshells are added. The second period includes eight elements because the second energy level (n=2) contains the 2s and 2p subshells, capable of holding a total of eight electrons.
The longer periods in the table (periods 4, 5, 6, and 7) are a consequence of the introduction of the d-block (transition metals) and the f-block (lanthanides and actinides). The d-block elements progressively fill the d orbitals, while the f-block elements fill the f orbitals. This expansion in subshells leads to the longer rows.
Trends Across a Period: A Systematic Analysis
Moving across a period from left to right, several important trends in the properties of elements emerge. These trends are closely related to the increase in the number of protons and electrons.
Atomic Radius: A Gradual Decrease
Atomic radius, the distance from the nucleus to the outermost electron, generally decreases as you move across a period. This is because the increasing nuclear charge (more protons) pulls the electrons closer to the nucleus, despite the addition of electrons to the same shell. The increased positive charge outweighs the effect of electron-electron repulsion, resulting in a smaller atomic size.
Ionization Energy: A General Increase
Ionization energy, the energy required to remove an electron from a neutral atom, generally increases across a period. The stronger nuclear pull exerted on the electrons makes it increasingly difficult to remove an electron, hence the rise in ionization energy.
Electronegativity: A Rising Trend
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. This is also a direct consequence of the increasing nuclear charge. Elements at the right-hand side of the period strongly attract electrons, exhibiting high electronegativity.
Metallic Character: A Decreasing Tendency
Metallic character, the tendency of an element to lose electrons and form positive ions, generally decreases across a period. As electronegativity increases, the ability of an element to lose electrons diminishes, leading to a decrease in metallic character. Elements on the left side of a period typically display strong metallic properties, while those on the right exhibit non-metallic characteristics.
Electron Affinity: A Complex Trend
Electron affinity, the energy change associated with adding an electron to a neutral atom, shows a more complex trend across a period. While there is a general increase, exceptions arise due to electron configurations and electron-electron repulsion within subshells.
Periods and Chemical Reactivity: Unveiling the Connections
The trends observed across a period directly influence the chemical reactivity of the elements.
Alkali Metals (Group 1): Highly Reactive
Alkali metals, located at the beginning of each period (except the first), have a single electron in their valence shell. This makes them highly reactive, readily losing this electron to form +1 ions. Their reactivity increases as you go down the group.
Halogens (Group 17): Reactive Nonmetals
Halogens, situated near the end of each period, have seven electrons in their valence shell. They are highly reactive nonmetals, readily gaining an electron to form -1 ions, thus achieving a stable octet configuration. Their reactivity decreases as you go down the group.
Noble Gases (Group 18): Inert Elements
Noble gases, found at the far right of each period, have a full valence shell (eight electrons, except for helium with two). This stable electron configuration makes them extremely unreactive, often termed "inert."
The Unique Nature of Transition Metals (d-block elements)
The transition metals, found in the d-block, represent a unique set of elements within the periodic table. Their properties differ from those of the main group elements (s-block and p-block) due to the filling of the d orbitals. Their variable oxidation states, contributing to a variety of chemical compounds, are a notable characteristic. The trends across a period within the d-block are less pronounced compared to the main group elements.
Periods and the Periodic Law: A Fundamental Principle
The periodic table is a testament to the periodic law, which states that the properties of elements are periodic functions of their atomic numbers. The repeating patterns observed in periods reinforce this law. The trends in atomic radius, ionization energy, electronegativity, and metallic character demonstrate the cyclical recurrence of chemical properties as you move across and down the table.
Practical Applications of Periodicity
Understanding the periodicity of elements is fundamental to various applications:
- Predicting chemical properties: Knowing the period of an element allows for predictions regarding its reactivity, bonding characteristics, and other chemical behaviors.
- Designing new materials: The periodic table helps in designing materials with specific properties based on the properties of the constituent elements.
- Understanding chemical reactions: Predicting the outcome of chemical reactions often relies on understanding the periodic trends and the reactivity of elements involved.
- Developing new technologies: The periodic table underpins many advancements in material science, electronics, and other technological fields.
Conclusion: The Indispensable Periods
Periods in the periodic table are not merely horizontal rows; they represent fundamental aspects of atomic structure and the systematic organization of elements. The trends observed across a period provide invaluable insights into the properties of elements and their chemical behavior. Understanding these trends is essential for anyone studying chemistry, whether a beginner or an experienced researcher. The periodic table, with its neatly organized periods and groups, remains a powerful tool for exploring the vast and complex world of chemistry, enabling predictions, explaining phenomena, and driving innovation. A strong grasp of the concept of periods forms a crucial foundation for understanding the intricate connections between atomic structure and chemical reactivity.
Latest Posts
Latest Posts
-
Digestion Of Food Is Chemical Or Physical Change
Mar 15, 2025
-
During The Energy Investment Phase Of Glycolysis
Mar 15, 2025
-
How Many Flat Surfaces Does A Rectangular Prism Have
Mar 15, 2025
-
Lowest Common Multiple Of 9 And 8
Mar 15, 2025
-
Real Life Examples Of Right Angles
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Horizontal Row In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
