What State Of Matter Takes The Shape Of Its Container
Juapaving
Mar 14, 2025 · 6 min read
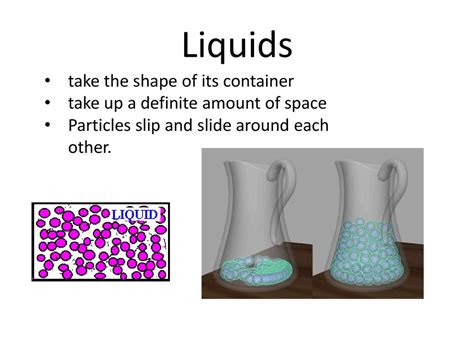
Table of Contents
What State of Matter Takes the Shape of Its Container? Understanding Fluids and Their Properties
The question of which state of matter takes the shape of its container is a fundamental concept in science, particularly in the study of fluids. While solids maintain a rigid shape, and gases expand to fill their entire container, the answer lies within the unique properties of liquids and gases. Both liquids and gases are classified as fluids due to their ability to flow and conform to the shape of their container. However, there are key differences in how they achieve this, and understanding these differences is crucial to comprehending their behavior and applications.
Liquids: The Conformists
Liquids are a state of matter characterized by a definite volume but an indefinite shape. This means that while the amount of liquid remains constant, its shape adapts to match the boundaries of its container. This adaptability stems from the nature of intermolecular forces within a liquid.
Intermolecular Forces and Liquid Shape
The molecules in a liquid are held together by relatively strong intermolecular forces, such as van der Waals forces, hydrogen bonds, and dipole-dipole interactions. These forces are weaker than the strong covalent or ionic bonds found in solids, allowing the molecules to move past one another. However, they're strong enough to maintain a relatively close proximity, resulting in a defined volume.
This balance between attractive forces and molecular motion is what permits liquids to flow and adapt to the shape of their container. Imagine pouring water into a glass. The water molecules, initially clustered together, readily rearrange themselves to fit the contours of the glass, filling the bottom and then rising to conform to the glass's shape.
Viscosity: Resistance to Flow
The ease with which a liquid conforms to its container is related to its viscosity. Viscosity is a measure of a fluid's resistance to flow. High-viscosity liquids, like honey or molasses, flow slowly and take longer to adopt the shape of their container. Low-viscosity liquids, like water, flow readily and conform to the container's shape quickly. Temperature significantly influences viscosity; heating a liquid typically reduces its viscosity, while cooling increases it.
Surface Tension: Maintaining Cohesion
Another important factor influencing the shape of a liquid is surface tension. Surface tension is the tendency of liquid surfaces to minimize their area. This force acts to keep the liquid molecules together, minimizing their contact with the air. It's responsible for phenomena like water droplets forming spherical shapes and insects walking on water. Although surface tension contributes to the overall cohesion of a liquid, it doesn't fundamentally dictate its ability to conform to the shape of a container. The primary factor remains the weaker intermolecular forces compared to solids.
Gases: The Universal Conformists
Gases are a state of matter with neither a definite volume nor a definite shape. Unlike liquids, which maintain a constant volume, gases expand to fill the entire volume of their container. This characteristic is a direct result of the weak intermolecular forces present in gases.
Weak Intermolecular Forces and Gas Expansion
The molecules in a gas are much farther apart than in a liquid or solid, and the intermolecular forces between them are incredibly weak. This allows the gas molecules to move freely and independently, constantly colliding with each other and the walls of their container. This constant motion and lack of significant attractive forces lead to the expansion of gases to fill any available space.
Pressure: A Consequence of Molecular Collisions
The pressure exerted by a gas is a direct consequence of the countless collisions its molecules make with the walls of the container. The more gas molecules present in a given volume, and the faster they are moving, the higher the pressure. This explains why gases conform to the shape of their container: the constant bombardment of molecules against the container walls forces the gas to conform to its boundaries.
Compressibility and Expansibility: Defining Characteristics
Gases are highly compressible and expansible. Compression involves reducing the volume of a gas by applying external pressure, bringing the gas molecules closer together. Expansion occurs when the external pressure is reduced or the gas is heated, allowing the gas molecules to move farther apart and occupy a larger volume. This extreme malleability explains the ease with which gases adapt to the shape and volume of their container.
Ideal Gas Law: A Mathematical Model
The behavior of gases is often described using the ideal gas law, a mathematical relationship that connects pressure, volume, temperature, and the number of moles of gas. The ideal gas law provides a simplified model for understanding how gases behave under various conditions, allowing for accurate predictions of their volume and pressure changes. While the ideal gas law is a simplification, it provides a strong framework for understanding the fundamentals of gas behavior. Deviations from ideal gas behavior are observed at high pressures and low temperatures, where intermolecular forces become more significant.
The Difference: Volume vs. Shape
The key difference between how liquids and gases conform to the shape of their containers lies in their behavior concerning volume. Liquids maintain a fixed volume, while gases do not. Liquids adapt their shape to the container while retaining their volume. Gases adapt both their shape and volume to fill the container completely. This difference stems from the strength of intermolecular forces: relatively weak forces in gases allow for complete volume adaptation, while stronger but still weaker than solids forces in liquids permit shape adaptation while preserving volume.
Real-World Examples
Understanding the ability of liquids and gases to conform to their containers is crucial in many aspects of our lives:
- Filling a water bottle: The water (liquid) conforms to the bottle's shape, filling the bottom and then rising to the top.
- Inflating a balloon: The air (gas) expands to completely fill the balloon, adopting both its shape and volume.
- Pouring soda: The soda (liquid) flows and takes the shape of the glass.
- Weather balloons: The helium (gas) inside the balloon expands as it rises in the atmosphere, increasing its volume to maintain equilibrium with the decreasing external pressure.
- Hydraulic systems: Liquids are used in hydraulic systems to transmit force, using their ability to conform to the shape of pipes and cylinders.
- Pneumatic systems: Compressed air (gas) is used in pneumatic systems to power tools and machinery.
Conclusion
Liquids and gases both conform to the shape of their container, but through different mechanisms. Liquids maintain a constant volume due to stronger intermolecular forces, adapting their shape to fit the container. Gases, with their weak intermolecular forces, expand to fill the entire volume of the container, adopting both its shape and volume. Understanding these fundamental differences and the interplay of intermolecular forces, viscosity, and pressure is crucial to comprehending the behavior of fluids and their applications across various scientific fields and engineering disciplines. This knowledge forms the basis of many technological advances, influencing everything from designing efficient hydraulic systems to predicting weather patterns based on gas dynamics. The ability of fluids to conform to their containers is a fundamental property that shapes our understanding of the physical world.
Latest Posts
Latest Posts
-
Does A Circle Have A Line Of Symmetry
Mar 14, 2025
-
How Many 3 6 Make One Whole
Mar 14, 2025
-
Digestion Of Food Physical Or Chemical Change
Mar 14, 2025
-
What Type Of Bond Is Nh3
Mar 14, 2025
-
What Type Of Bond Holds Nitrogen Bases Together
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What State Of Matter Takes The Shape Of Its Container . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
