What Type Of Bond Is Nh3
Juapaving
Mar 14, 2025 · 6 min read
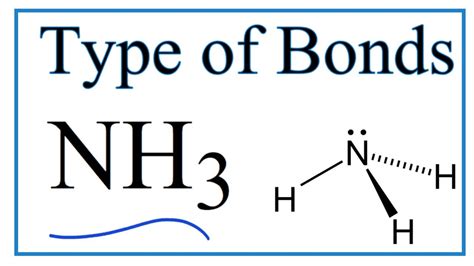
Table of Contents
What Type of Bond is NH₃? A Deep Dive into Ammonia's Molecular Structure and Bonding
Ammonia (NH₃), a colorless gas with a pungent odor, plays a crucial role in various industrial processes and biological systems. Understanding its bonding characteristics is fundamental to comprehending its properties and reactivity. This article delves deep into the type of bond present in NH₃, exploring its molecular geometry, polarity, and the implications of its bonding for its chemical behavior.
The Covalent Nature of NH₃ Bonds
The primary type of bond in ammonia is a covalent bond. This is because nitrogen (N) and hydrogen (H) atoms share electrons to achieve a stable electron configuration. Unlike ionic bonds, where electrons are transferred from one atom to another, covalent bonds involve the sharing of electrons between atoms. This sharing creates a strong attractive force that holds the atoms together, forming the NH₃ molecule.
Understanding Electron Sharing in NH₃
Nitrogen, located in Group 15 of the periodic table, has five valence electrons. To achieve a stable octet (eight valence electrons), it needs to gain three more electrons. Hydrogen, in Group 1, has one valence electron and needs one more electron to achieve a stable duet (two valence electrons).
In ammonia, each hydrogen atom shares its single valence electron with the nitrogen atom. Simultaneously, the nitrogen atom shares one of its electrons with each hydrogen atom. This results in three covalent bonds, each composed of a shared electron pair. The nitrogen atom now has eight electrons in its valence shell (three shared pairs and one lone pair), satisfying the octet rule, while each hydrogen atom has two electrons, satisfying the duet rule.
Representing NH₃ Bonding: Lewis Structures and VSEPR Theory
Lewis structures provide a simple way to visualize the bonding in molecules. In NH₃'s Lewis structure, the nitrogen atom is in the center, surrounded by three hydrogen atoms. Three lines represent the three covalent bonds, and two dots represent the lone pair of electrons on the nitrogen atom.
The Valence Shell Electron Pair Repulsion (VSEPR) theory helps predict the three-dimensional geometry of molecules based on the repulsion between electron pairs in the valence shell. In NH₃, the four electron pairs (three bonding pairs and one lone pair) arrange themselves in a tetrahedral geometry to minimize repulsion. However, the molecular geometry, considering only the positions of the atoms, is trigonal pyramidal. This means the molecule has a pyramid shape with the nitrogen atom at the apex and the three hydrogen atoms forming the base.
Polarity of N-H Bonds and the Ammonia Molecule
The N-H bonds in ammonia are polar covalent bonds. This is due to the electronegativity difference between nitrogen and hydrogen. Nitrogen is more electronegative than hydrogen, meaning it attracts the shared electrons in the N-H bonds more strongly. This results in a partial negative charge (δ-) on the nitrogen atom and partial positive charges (δ+) on the hydrogen atoms.
The overall molecule of NH₃ is also polar. While the individual bond dipoles don't perfectly cancel each other out due to the trigonal pyramidal geometry, the resultant dipole moment is significant. This polarity significantly impacts ammonia's properties, including its solubility in water and its ability to act as a weak base.
Implications of Polarity for Ammonia's Properties
The polarity of NH₃ is responsible for several key properties:
-
High boiling point: Compared to other molecules of similar molecular weight, ammonia has a relatively high boiling point. This is due to the strong hydrogen bonding between ammonia molecules. The partially positive hydrogen atoms of one ammonia molecule are attracted to the partially negative nitrogen atoms of neighboring molecules. This intermolecular attraction requires more energy to overcome, leading to a higher boiling point.
-
Solubility in water: Ammonia is highly soluble in water. This is attributed to the hydrogen bonding between ammonia and water molecules. The partially positive hydrogen atoms of water molecules form hydrogen bonds with the partially negative nitrogen atom of ammonia, and vice versa.
-
Weak base properties: Ammonia acts as a weak base, readily accepting protons (H⁺) from acids. The lone pair of electrons on the nitrogen atom can readily accept a proton, forming the ammonium ion (NH₄⁺). This property is a direct consequence of the nitrogen's partially negative charge and the availability of the lone pair.
Comparison with Other Bond Types
It's essential to understand how covalent bonds in NH₃ differ from other bond types:
-
Ionic Bonds: Ionic bonds form between atoms with significantly different electronegativities, leading to a complete transfer of electrons. In contrast, covalent bonds involve electron sharing. The electronegativity difference between N and H is not large enough to form an ionic bond.
-
Metallic Bonds: Metallic bonds occur in metals, where valence electrons are delocalized across a lattice of metal atoms. This type of bonding is not present in NH₃, a covalent molecule.
-
Coordinate Covalent Bonds (Dative Bonds): In a coordinate covalent bond, both electrons in the shared pair come from the same atom. While not directly present in the initial formation of NH₃, ammonia can participate in coordinate covalent bonds, for example, when forming the ammonium ion (NH₄⁺) by accepting a proton from an acid. The proton contributes both electrons to form the new N-H bond.
The Significance of Ammonia's Bonding in Various Applications
The unique bonding characteristics of ammonia underpin its widespread applications:
-
Fertilizers: Ammonia is a crucial component in the production of nitrogen-based fertilizers. Its ability to readily donate nitrogen to plants makes it an essential nutrient source for agriculture.
-
Refrigerants: Ammonia's high heat of vaporization makes it an effective refrigerant. Its use in refrigeration systems is becoming increasingly popular due to its environmentally friendly nature compared to some other refrigerants.
-
Industrial Cleaning: Ammonia is used as a cleaning agent due to its ability to dissolve grease and grime. However, caution must be exercised as it is corrosive and irritating.
-
Pharmaceuticals and Dyes: Ammonia is used as an intermediate in the synthesis of various pharmaceuticals and dyes. Its reactivity with diverse compounds stems from its unique bonding characteristics.
-
Biological Systems: Ammonia plays a crucial role in biological systems. It is a product of protein metabolism and is a key component in the synthesis of amino acids and nucleotides. Furthermore, the ammonium ion is essential for the acid-base balance in living organisms.
Conclusion
In summary, the bonds in NH₃ are polar covalent bonds. The nitrogen atom shares electrons with three hydrogen atoms, forming three covalent bonds and resulting in a trigonal pyramidal molecular geometry. The polarity of these bonds and the presence of a lone pair on the nitrogen atom are responsible for ammonia's characteristic properties, such as its high boiling point, solubility in water, and ability to act as a weak base. These properties, in turn, determine its vast array of applications in industry, agriculture, and biological systems. Understanding the nature of the bonding in NH₃ is vital for appreciating its significance in the world around us. Further exploration of its reactivity and interactions with other molecules provides a more comprehensive understanding of this crucial compound.
Latest Posts
Latest Posts
-
What Shape Has 6 Equal Faces
Mar 14, 2025
-
Water Is Good Conductor Of Heat
Mar 14, 2025
-
What Is 1 625 As A Fraction
Mar 14, 2025
-
Differentiate Between Empirical And Molecular Formula
Mar 14, 2025
-
Least Common Multiple Of 20 And 24
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Is Nh3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
