What Remains Constant In Charles Law
Juapaving
Mar 08, 2025 · 6 min read
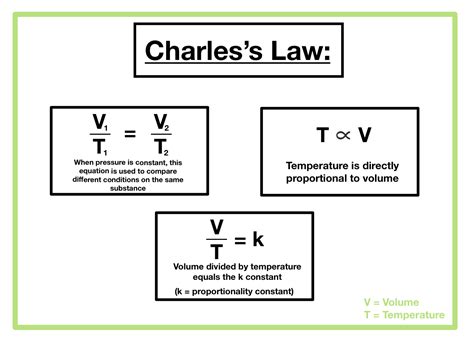
Table of Contents
What Remains Constant in Charles's Law: A Deep Dive into Volume-Temperature Relationships
Charles's Law, a cornerstone of ideal gas behavior, describes the direct relationship between the volume and temperature of a gas when pressure and the amount of gas are held constant. While the law itself is elegantly simple, a thorough understanding requires delving into what truly remains constant and the implications of those constants. This article will explore the fundamental conditions of Charles's Law, examining the underlying principles, limitations, and practical applications.
The Invariable Trio: Pressure, Amount of Gas, and the Ideal Gas Assumption
Charles's Law, often expressed as V₁/T₁ = V₂/T₂, hinges on three crucial constants:
1. Constant Pressure: The Unwavering Force
Maintaining a constant pressure is paramount. Imagine a gas sample contained in a flexible container. If the temperature increases, the gas molecules move faster, colliding more frequently and forcefully with the container walls. If the pressure were allowed to change, the container would expand or contract, invalidating the law's premise. Only by keeping the pressure stable, preventing any changes in the force exerted by the gas per unit area, can we observe the direct relationship between volume and temperature as described by Charles's Law. This constancy is achieved through various experimental setups, ensuring the external pressure remains uniform throughout the process.
2. Constant Amount of Gas: No Leaks, No Additions
The number of gas molecules within the system must remain unchanged. This means no gas can leak out, nor can any additional gas be introduced. The integrity of the container is critical; any leaks would alter the gas quantity, disrupting the observed relationship between volume and temperature. This constancy ensures that the observed changes in volume are solely attributed to temperature variations, not the addition or removal of gas particles. Maintaining a sealed system is therefore crucial for accurate experimental results.
3. The Ideal Gas Assumption: A Simplification for Clarity
Charles's Law is fundamentally based on the ideal gas law, PV = nRT. This equation assumes that gas molecules have negligible volume and exert no intermolecular forces. While no real gas perfectly fits this description, many gases behave approximately ideally under certain conditions (low pressure and high temperature). The assumption of ideal gas behavior simplifies the analysis, allowing for a direct and easily measurable relationship between volume and temperature. However, it's crucial to remember that deviations from ideal behavior occur at high pressures and low temperatures where intermolecular forces and molecular volume become significant. These deviations will be discussed in greater detail later.
The Relationship: Direct Proportionality and Absolute Zero
The core of Charles's Law lies in the direct proportionality between volume and absolute temperature. This means that if the absolute temperature doubles, the volume will also double, provided pressure and the amount of gas remain constant. The absolute temperature, measured in Kelvin (K), is crucial. Unlike Celsius or Fahrenheit, the Kelvin scale has a true zero point—absolute zero—representing the theoretical temperature at which all molecular motion ceases. This is why Charles's Law is expressed using Kelvin: it provides a consistent and meaningful scale for the relationship.
Using the Kelvin scale avoids the complications of negative temperatures that could arise if Celsius were used. A negative volume is physically impossible. Expressing temperature in Kelvin ensures that the volume and temperature always have the same sign, reflecting the direct proportionality.
Beyond the Equation: Microscopic Insights
Charles's Law's macroscopic observations can be explained microscopically:
- Increased Temperature, Increased Kinetic Energy: When the temperature increases, the kinetic energy of the gas molecules increases. They move faster and more vigorously.
- Increased Collisions, Increased Volume: The increased kinetic energy leads to more frequent and forceful collisions with the container walls. To maintain constant pressure, the container must expand, increasing the volume to accommodate the more energetic molecules. The expansion counteracts the increased force of collisions, maintaining a constant pressure.
- Decreased Temperature, Decreased Kinetic Energy: Conversely, a decrease in temperature reduces the kinetic energy of gas molecules. They move slower, resulting in less frequent and less forceful collisions. The container will contract, reducing the volume to maintain constant pressure.
Limitations and Deviations from Ideal Behavior
While Charles's Law provides a useful approximation for many gases under ordinary conditions, it's essential to acknowledge its limitations:
- Non-Ideal Gases: At high pressures or low temperatures, real gases deviate significantly from ideal behavior. Intermolecular forces become substantial, and the volume occupied by the gas molecules themselves becomes appreciable. These factors affect the relationship between volume and temperature, causing deviations from the predictions of Charles's Law.
- Critical Point: Every gas has a critical point, a temperature and pressure above which it cannot be liquefied. Near the critical point, intermolecular forces become dominant, rendering Charles's Law inapplicable.
- Chemical Reactions: If the gas undergoes a chemical reaction, the number of gas molecules changes. This violates the constant amount of gas condition, making Charles's Law invalid.
Practical Applications: Real-World Uses
Despite its limitations, Charles's Law finds applications in various fields:
- Hot Air Balloons: The principle of Charles's Law is directly responsible for the flight of hot air balloons. Heating the air inside the balloon increases its volume, making it less dense than the surrounding cooler air, thereby providing buoyancy.
- Weather Forecasting: Understanding the relationship between temperature and volume helps meteorologists predict weather patterns and understand atmospheric phenomena.
- Automotive Engineering: Charles's Law plays a role in designing automotive engine systems, particularly those involving the intake and exhaust of gases.
- Aerospace Engineering: The behaviour of gases at different altitudes and temperatures is crucial in aircraft design and operation. Charles's Law contributes to calculations involving gas expansion and contraction.
- Refrigeration and Air Conditioning: Refrigeration systems rely on the expansion and contraction of gases as their temperature changes, a principle directly linked to Charles's Law.
Conclusion: A Fundamental Principle with Practical Implications
Charles's Law, while based on the simplification of ideal gas behavior, provides a valuable framework for understanding the relationship between the volume and temperature of a gas under constant pressure and amount of gas. The constancy of pressure and amount of gas, along with the reliance on the ideal gas assumption, are crucial in understanding the limitations and applicability of this fundamental principle of gas behaviour. Understanding these constants, their implications, and the limitations of the law allows for accurate predictions and effective applications across various scientific and engineering disciplines. By appreciating the microscopic basis of the law and recognizing its deviations under non-ideal conditions, we can apply Charles's Law effectively while remaining aware of its boundaries. The interplay between theory and application underlines the significance of this important scientific principle.
Latest Posts
Latest Posts
-
How Many Sides Are On A Octagon
Mar 09, 2025
-
What Is The Prime Factorization Of 180
Mar 09, 2025
-
How Many Sides Are In A Quadrilateral
Mar 09, 2025
-
How Are Molecules Different From Compounds
Mar 09, 2025
-
What Is The Factor Of 31
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Remains Constant In Charles Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
