How Are Molecules Different From Compounds
Juapaving
Mar 09, 2025 · 6 min read
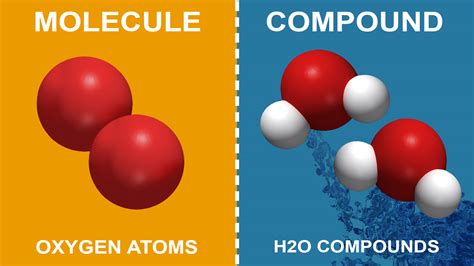
Table of Contents
How Are Molecules Different From Compounds? A Deep Dive into Chemical Structures
The terms "molecule" and "compound" are frequently used interchangeably in casual conversation, leading to confusion about their distinct meanings in chemistry. While closely related, they represent fundamentally different concepts in the structure and composition of matter. Understanding their differences is crucial for grasping the basics of chemistry and appreciating the complexity of the molecular world. This comprehensive guide will delve into the nuances of molecules and compounds, clarifying their definitions, exploring their properties, and highlighting key distinctions through examples.
Defining Molecules: The Building Blocks of Matter
A molecule is a group of two or more atoms held together by chemical bonds. These atoms can be of the same element (like in the case of oxygen gas, O₂), or they can be from different elements (like in water, H₂O). The key characteristic of a molecule is its discrete nature; it's a distinct entity with a specific arrangement of atoms. The bonds within a molecule determine its shape, size, and properties. These bonds can be covalent bonds, where atoms share electrons, or ionic bonds, where one atom donates an electron to another, creating oppositely charged ions that attract each other.
Types of Molecules: A Diverse World
Molecules exist in a staggering array of forms, varying vastly in size, complexity, and functionality. From simple diatomic molecules like hydrogen (H₂) and nitrogen (N₂) to gigantic macromolecules like proteins and DNA, the diversity is immense.
1. Diatomic Molecules: These molecules consist of just two atoms of the same element. Examples include oxygen (O₂), nitrogen (N₂), hydrogen (H₂), fluorine (F₂), chlorine (Cl₂), bromine (Br₂), and iodine (I₂). These elements exist naturally as diatomic molecules due to the stability gained by sharing electrons.
2. Triatomic and Polyatomic Molecules: These molecules contain three or more atoms, which can be of the same or different elements. Water (H₂O) is a simple example of a triatomic molecule, while ozone (O₃) is another. Larger polyatomic molecules can become extremely complex, with thousands of atoms arranged in intricate structures.
3. Macromolecules: These are large, complex molecules composed of many smaller subunits, often linked together in chains or other repeating patterns. Proteins, carbohydrates, lipids, and nucleic acids (DNA and RNA) are prime examples of macromolecules essential for life.
Defining Compounds: Molecules with Multiple Elements
A compound is a substance formed when two or more different chemical elements are chemically bonded together. This is a crucial distinction: a compound must involve at least two different elements. The elements in a compound are always present in a definite proportion by mass. This means that the ratio of the different elements in a compound is always consistent, regardless of the size or source of the compound. This is expressed by a chemical formula (e.g., H₂O for water, NaCl for table salt).
Properties of Compounds: Unique Identities
The properties of a compound are often very different from the properties of the elements that make it up. For instance, sodium (Na) is a highly reactive metal, and chlorine (Cl) is a toxic gas. However, their compound, sodium chloride (NaCl), or common table salt, is a relatively unreactive and essential nutrient. This dramatic difference in properties highlights the transformative nature of chemical bonding.
Classifying Compounds: An Organizational Framework
Compounds are further classified based on their chemical bonding and properties. Two major categories include:
1. Ionic Compounds: These compounds are formed through ionic bonds, where one or more electrons are transferred from one atom to another, creating oppositely charged ions that are held together by electrostatic attraction. Table salt (NaCl) is a classic example of an ionic compound. They often have high melting points and dissolve readily in water.
2. Covalent Compounds: These compounds are formed through covalent bonds, where atoms share electrons to achieve a more stable electron configuration. Water (H₂O) and methane (CH₄) are examples of covalent compounds. They often have lower melting points compared to ionic compounds and may or may not dissolve in water.
Key Differences Between Molecules and Compounds
While all compounds are molecules, not all molecules are compounds. This seemingly contradictory statement encapsulates the core difference:
| Feature | Molecule | Compound |
|---|---|---|
| Definition | Two or more atoms bonded together | Two or more different elements bonded together |
| Elements | Can be same or different elements | Must be at least two different elements |
| Examples | O₂, H₂, H₂O, CO₂, C₆H₁₂O₆ | H₂O, NaCl, CO₂, C₆H₁₂O₆ |
| Properties | Properties depend on the atoms & bonds | Properties differ significantly from constituent elements |
In essence:
- All compounds are molecules, but not all molecules are compounds. A molecule is a more general term referring to any collection of atoms bonded together. A compound is a specific type of molecule consisting of at least two different elements.
Examples to Illustrate the Distinction
Let's clarify the difference with some concrete examples:
-
Oxygen (O₂): This is a molecule because it consists of two oxygen atoms bonded together. However, it's not a compound because it only contains one type of element.
-
Water (H₂O): This is both a molecule and a compound. It's a molecule because it consists of atoms bonded together (two hydrogen atoms and one oxygen atom). It's a compound because it contains two different elements (hydrogen and oxygen).
-
Carbon Dioxide (CO₂): This is both a molecule and a compound, containing carbon and oxygen atoms.
-
Glucose (C₆H₁₂O₆): This is both a molecule (a sugar molecule) and a compound (composed of carbon, hydrogen, and oxygen).
-
Sodium Chloride (NaCl): This is both a molecule and a compound, composed of sodium and chlorine ions.
Beyond the Basics: Exploring Advanced Concepts
Understanding the distinction between molecules and compounds is foundational. As you delve deeper into chemistry, this understanding will become increasingly crucial for comprehending:
-
Stoichiometry: The quantitative relationships between reactants and products in chemical reactions heavily rely on understanding molecular and compound formulas.
-
Chemical Reactions: Reactions involve the breaking and forming of chemical bonds within molecules and compounds, leading to the formation of new substances.
-
Organic Chemistry: The study of carbon-containing compounds heavily relies on understanding the diverse structures and functionalities of organic molecules.
-
Biochemistry: The study of life's chemical processes is fundamentally based on the structure and interactions of biological molecules, including proteins, carbohydrates, lipids, and nucleic acids.
Conclusion: Mastering the Molecular Landscape
The seemingly simple distinction between molecules and compounds forms the bedrock of chemical understanding. While the terms are often used interchangeably in everyday language, their precise scientific meanings are critical for accurate communication and comprehension of chemical phenomena. By mastering this fundamental concept, you unlock a deeper appreciation of the incredibly complex and fascinating world of molecules and compounds. This understanding provides a solid foundation for further exploration of chemistry's intricate details and its pivotal role in our world.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 8 And 3
Mar 09, 2025
-
Moment Of Inertia Of A Semicircle
Mar 09, 2025
-
Where Does Dna Replication Occur In Eukaryotic Cells
Mar 09, 2025
-
How Many Atoms Are In Chlorine
Mar 09, 2025
-
What Is The First Five Multiples Of 9
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How Are Molecules Different From Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
