What Is The Standard Electrode Potential
Juapaving
Mar 28, 2025 · 6 min read
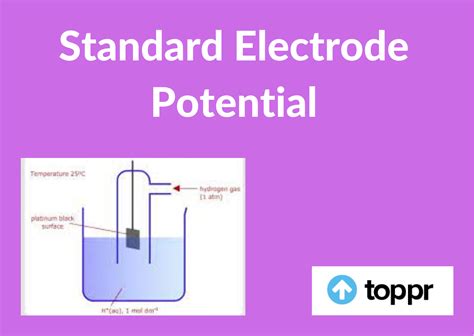
Table of Contents
What is Standard Electrode Potential? A Comprehensive Guide
Standard electrode potential, often represented as E⁰, is a crucial concept in electrochemistry. It quantifies the relative tendency of a half-cell to undergo reduction, providing a powerful tool for predicting the spontaneity of redox reactions. Understanding standard electrode potential is key to comprehending various electrochemical processes, from battery operation to corrosion prevention. This comprehensive guide will delve into its definition, measurement, applications, and limitations.
Understanding the Fundamentals: Half-Cells and Reduction Potentials
Before delving into standard electrode potential, let's establish a solid foundation. Electrochemical reactions involve the transfer of electrons between species. These reactions are often broken down into two half-reactions: one involving oxidation (loss of electrons) and the other involving reduction (gain of electrons). Each half-reaction occurs in a separate compartment called a half-cell.
A half-cell typically consists of a metal electrode immersed in a solution containing its ions. For example, a zinc half-cell might consist of a zinc electrode (Zn) in a solution of zinc sulfate (ZnSO₄). The electrode's potential, relative to a reference electrode, indicates its tendency to undergo reduction. A higher reduction potential signifies a greater tendency to gain electrons, meaning the species is a stronger oxidizing agent.
Defining Standard Electrode Potential (E⁰)
The standard electrode potential, E⁰, measures the potential difference between a half-cell and a standard hydrogen electrode (SHE) under standard conditions. These conditions include:
- Temperature: 298 K (25°C)
- Pressure: 1 atmosphere (101.325 kPa)
- Concentration: 1 molar (1 M) for all aqueous solutions
The SHE serves as the reference point, with its potential arbitrarily assigned as 0 volts. Therefore, the standard electrode potential of a half-cell represents the electromotive force (EMF) generated when that half-cell is connected to the SHE. A positive E⁰ value indicates that the half-reaction has a greater tendency to undergo reduction than the reduction of H⁺ ions in the SHE. Conversely, a negative E⁰ value suggests the half-reaction is less likely to undergo reduction than the SHE reaction.
Measuring Standard Electrode Potential: The Voltaic Cell
Measuring E⁰ requires constructing a voltaic cell (also known as a galvanic cell), which involves connecting two half-cells via a salt bridge. The salt bridge allows the flow of ions to maintain electrical neutrality, completing the circuit. The potential difference measured between the two electrodes represents the cell potential (Ecell).
To determine the standard electrode potential of a specific half-cell, it is paired with the SHE. The measured cell potential is then directly equal to the standard electrode potential of the other half-cell because the SHE's potential is zero. For example, if a cell composed of a zinc half-cell and a SHE yields a cell potential of -0.76 V, the standard electrode potential of the zinc half-cell (Zn²⁺ + 2e⁻ → Zn) is -0.76 V.
Applications of Standard Electrode Potential
Standard electrode potentials have numerous applications in various fields:
1. Predicting the Spontaneity of Redox Reactions:
The most significant application of E⁰ is predicting the spontaneity of redox reactions. The overall cell potential (E°cell) of a redox reaction is calculated by subtracting the standard electrode potential of the anode (oxidation half-reaction) from the standard electrode potential of the cathode (reduction half-reaction):
E°cell = E°cathode - E°anode
A positive E°cell indicates that the reaction will proceed spontaneously under standard conditions. A negative E°cell signifies a non-spontaneous reaction under standard conditions. This principle is crucial in designing electrochemical cells, understanding corrosion processes, and analyzing metabolic pathways.
2. Determining the Equilibrium Constant:
The standard cell potential is related to the equilibrium constant (K) of the redox reaction via the Nernst equation:
E°cell = (RT/nF)lnK
Where:
- R is the ideal gas constant
- T is the temperature in Kelvin
- n is the number of electrons transferred in the balanced redox reaction
- F is Faraday's constant
This relationship allows for the determination of the equilibrium constant from the measured standard cell potential, providing insights into the extent to which the reaction will proceed to completion.
3. Corrosion Prediction and Prevention:
Standard electrode potentials are instrumental in understanding and preventing corrosion. Corrosion is essentially an electrochemical process involving oxidation of a metal. By comparing the standard electrode potentials of different metals, one can predict which metals are more prone to corrosion in a given environment. This knowledge facilitates the selection of appropriate materials for specific applications and the implementation of corrosion prevention techniques, such as cathodic protection.
4. Electroplating and Electrosynthesis:
Electroplating and electrosynthesis are electrochemical processes that rely heavily on standard electrode potentials. These processes involve using an electric current to deposit a thin layer of metal onto a surface (electroplating) or to synthesize specific compounds (electrosynthesis). The choice of electrode materials and the applied potential are guided by the standard electrode potentials of the involved species.
5. Battery Design and Performance:
Standard electrode potentials play a critical role in designing and optimizing battery performance. Batteries consist of two half-cells with differing standard electrode potentials. The difference in potentials determines the voltage and overall energy capacity of the battery. The selection of appropriate electrode materials and electrolytes is guided by their standard electrode potentials to ensure optimal battery performance and longevity.
Limitations of Standard Electrode Potential
While standard electrode potentials are incredibly useful, it's important to acknowledge their limitations:
- Standard Conditions: E⁰ values are only valid under standard conditions (298 K, 1 atm, 1 M). Deviations from these conditions will affect the actual cell potential. The Nernst equation is used to calculate the cell potential under non-standard conditions.
- Activity vs. Concentration: The Nernst equation ideally uses activities rather than concentrations. Activity accounts for interionic interactions, which can significantly affect the potential, especially at higher concentrations.
- Kinetic Factors: E⁰ only indicates the thermodynamic feasibility of a reaction; it doesn't provide information about the reaction rate. Some reactions may be thermodynamically favorable but kinetically slow.
- Complex Reactions: The use of E⁰ becomes more challenging for complex reactions involving multiple steps or intermediates. It may not accurately reflect the overall reaction's potential.
- Overpotential: Overpotential, the additional voltage required to drive an electrochemical reaction beyond the thermodynamically predicted value, is not considered in standard electrode potentials. This is particularly significant in industrial electrochemical processes.
Conclusion: The Significance of Standard Electrode Potential in Electrochemistry
Standard electrode potential (E⁰) serves as a cornerstone of electrochemistry, providing a quantitative measure of a half-cell's reduction tendency. Its applications are far-reaching, impacting various fields from predicting reaction spontaneity to designing efficient batteries and preventing corrosion. While acknowledging its limitations is crucial for accurate interpretation, understanding standard electrode potential remains essential for comprehending and manipulating electrochemical processes. The information provided in this guide offers a comprehensive understanding of this vital concept, empowering you to delve deeper into the fascinating world of electrochemistry. Further research into specific applications and variations from standard conditions will provide an even more nuanced understanding of this critical electrochemical parameter.
Latest Posts
Latest Posts
-
Formic Acid Is A Weak Acid
Mar 31, 2025
-
Mass Of An Electron In Mev
Mar 31, 2025
-
From A Gas To A Liquid
Mar 31, 2025
-
How Many Feet Is 16 Meters
Mar 31, 2025
-
Examples Of Gas To A Liquid
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Standard Electrode Potential . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
