From A Gas To A Liquid
Juapaving
Mar 31, 2025 · 6 min read
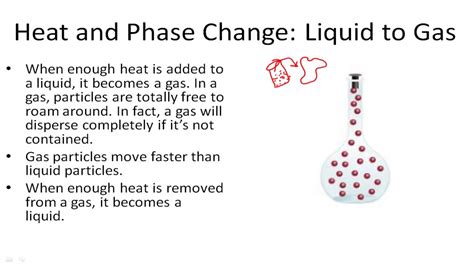
Table of Contents
From Gas to Liquid: A Comprehensive Guide to Condensation
The transformation of a gas into a liquid, a process known as condensation, is a fundamental concept in chemistry and physics with far-reaching implications in various fields. Understanding this process requires delving into the behavior of molecules, the role of intermolecular forces, and the influence of external factors like temperature and pressure. This comprehensive guide will explore the intricacies of condensation, covering its underlying mechanisms, practical applications, and real-world examples.
Understanding the Gaseous State
Before delving into the process of condensation, it's crucial to establish a firm understanding of the gaseous state. Gases are characterized by their lack of definite shape and volume. Their molecules are widely dispersed, possessing high kinetic energy, leading to constant, random motion. This movement results in weak intermolecular forces – the attractions between individual gas molecules. The strength of these forces plays a critical role in determining the conditions under which a gas will condense.
Kinetic Molecular Theory and Gas Behavior
The Kinetic Molecular Theory (KMT) provides a framework for understanding gas behavior. This theory posits that gases consist of tiny particles (atoms or molecules) in constant, random motion. These particles are considered to have negligible volume compared to the volume of the container they occupy and exert no significant attractive or repulsive forces on each other, except during collisions. The pressure exerted by a gas is a result of these countless collisions with the container walls.
Intermolecular Forces: The Key to Condensation
While KMT simplifies gas behavior, it doesn't fully account for the real-world behavior of all gases. In reality, attractive forces exist between gas molecules, albeit weak compared to the forces within molecules. These intermolecular forces, such as van der Waals forces (including London dispersion forces, dipole-dipole interactions, and hydrogen bonding), are crucial for understanding condensation. These forces are responsible for the attraction between molecules, pulling them closer together.
The Condensation Process: From Chaos to Order
Condensation occurs when a gas transitions to a liquid state. This transition involves a decrease in kinetic energy of the gas molecules. As the molecules slow down, the intermolecular forces become more significant. These attractive forces overcome the kinetic energy of the molecules, causing them to clump together and form a liquid.
The Role of Temperature and Pressure
Temperature and pressure are the primary factors influencing condensation.
-
Temperature: Lowering the temperature reduces the kinetic energy of gas molecules. This weakens their ability to overcome intermolecular forces, making condensation more likely. When the temperature falls below the dew point, the gas begins to condense. The dew point is the temperature at which the air becomes saturated with water vapor, and further cooling leads to condensation.
-
Pressure: Increasing the pressure forces gas molecules closer together, increasing the frequency of intermolecular interactions. This enhances the attractive forces between molecules, facilitating condensation. Higher pressure effectively reduces the volume available to the gas molecules, leading to increased interaction and eventual condensation.
Condensation and Phase Diagrams
Phase diagrams visually represent the relationship between temperature, pressure, and the phases of matter (solid, liquid, gas). These diagrams show the conditions under which a substance exists in different phases. The line separating the gas and liquid phases on a phase diagram represents the conditions under which condensation can occur. The point where the three phases (solid, liquid, gas) coexist is called the triple point.
Mechanisms of Condensation
Condensation occurs through various mechanisms, depending on the specific conditions and the nature of the gas.
Homogeneous Nucleation
In homogeneous nucleation, condensation occurs spontaneously within the gas phase itself. This requires the formation of tiny liquid droplets within the gas, which serve as nuclei for further condensation. This process is typically more challenging as it requires overcoming a significant energy barrier.
Heterogeneous Nucleation
Heterogeneous nucleation is a more common process, particularly in the atmosphere. Here, condensation occurs on surfaces, such as dust particles, aerosols, or other surfaces. These surfaces provide pre-existing sites for the gas molecules to condense onto, reducing the energy barrier for condensation. This is why clouds form around tiny particles in the atmosphere.
Practical Applications of Condensation
Condensation plays a vital role in numerous practical applications across various industries.
Dew Formation
The formation of dew is a classic example of condensation. During cool nights, the temperature of surfaces (like grass or leaves) falls below the dew point of the surrounding air. The water vapor in the air then condenses onto these surfaces, forming dew.
Fog and Cloud Formation
Fog and clouds are large-scale manifestations of condensation. Water vapor in the atmosphere condenses onto microscopic particles, forming tiny water droplets or ice crystals that we perceive as fog or clouds.
Refrigeration and Air Conditioning
Refrigeration and air conditioning systems utilize condensation to cool spaces. These systems utilize refrigerants that undergo phase transitions from gas to liquid, releasing heat in the process, thereby cooling the surrounding environment.
Distillation
Distillation is a separation technique that relies on the differences in boiling points of various components in a liquid mixture. Condensation is crucial in this process, as it allows for the collection of the separated components as liquids.
Liquefaction of Gases
Many industrial processes require the liquefaction of gases, such as natural gas or air. This is achieved by cooling and compressing the gas until it condenses into a liquid. Liquefied gases are easier to store and transport than their gaseous counterparts.
Real-World Examples and Case Studies
Numerous real-world phenomena showcase the significance of condensation.
Rainfall
Rainfall is a quintessential example of condensation on a massive scale. Water vapor in the atmosphere condenses to form clouds, and when these droplets become sufficiently large, they fall as rain. The process of rainfall is intricate and depends on various atmospheric conditions, including temperature, pressure, and the presence of condensation nuclei.
Breath on a Cold Mirror
The condensation of water vapor from your breath on a cold mirror is a readily observable example. The warm, moist air from your breath comes into contact with the cold surface of the mirror, causing the water vapor to condense into tiny water droplets, forming a visible film.
Formation of Frost
Frost is formed through deposition, a process where water vapor directly transitions from a gas to a solid (ice) without passing through the liquid phase. While not strictly condensation, it is a closely related phase transition influenced by similar factors like temperature and humidity.
Conclusion
Condensation, the transition from a gas to a liquid, is a pervasive phenomenon underpinning numerous natural processes and technological applications. Understanding the fundamental principles of condensation—the role of intermolecular forces, temperature, pressure, and nucleation—is crucial for comprehending a wide range of scientific and engineering concepts. From the formation of dew to the operation of refrigeration systems and the production of liquefied gases, condensation’s importance is undeniable. This comprehensive overview provides a solid foundation for exploring the intricacies of this vital phase transition. Further research into specific aspects, such as the behavior of different gases, the influence of specific surface properties on nucleation, and the impact of climate change on condensation processes, will undoubtedly yield even deeper insights into this fascinating and multifaceted phenomenon.
Latest Posts
Latest Posts
-
What Is The Only Movable Bone Of The Skull
Apr 02, 2025
-
How Many Inches Are In One Meter
Apr 02, 2025
-
What Is The Freezing Point Of Water In Kelvin Scale
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about From A Gas To A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
