What Is The Molecular Mass Of Calcium Nitrate
Juapaving
Mar 24, 2025 · 6 min read
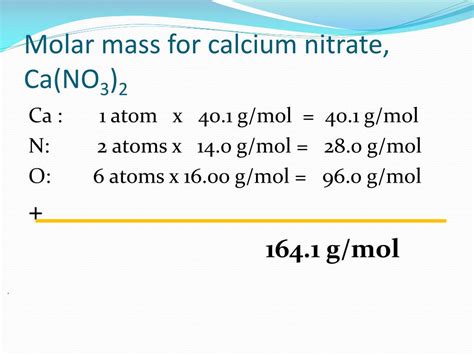
Table of Contents
What is the Molecular Mass of Calcium Nitrate? A Deep Dive into Chemical Calculations
Determining the molecular mass (or molar mass) of a compound is a fundamental skill in chemistry. Understanding this concept is crucial for various applications, from stoichiometric calculations to solution preparation. This article delves into the process of calculating the molecular mass of calcium nitrate (Ca(NO₃)₂), exploring the underlying principles and providing a step-by-step guide. We'll also explore related concepts and practical applications.
Understanding Molecular Mass
Molecular mass, often used interchangeably with molar mass, represents the mass of one mole of a substance. A mole is a unit in the International System of Units (SI) representing a specific number of particles – Avogadro's number (approximately 6.022 x 10²³). Therefore, the molecular mass tells us the mass of 6.022 x 10²³ molecules or formula units of a given substance in grams. This mass is numerically equal to the sum of the atomic masses of all the atoms in the chemical formula, expressed in atomic mass units (amu) or grams per mole (g/mol).
Atomic Mass and the Periodic Table
The foundation of molecular mass calculation lies in the atomic masses of individual elements. These values are found on the periodic table. Each element's atomic mass reflects the average mass of its isotopes, weighted by their relative abundances in nature. For example, the atomic mass of carbon (C) is approximately 12.01 amu, representing the average mass of carbon-12 and carbon-13 isotopes.
Calculating the Molecular Mass of Calcium Nitrate Ca(NO₃)₂
Calcium nitrate, Ca(NO₃)₂, is an ionic compound composed of calcium (Ca²⁺) cations and nitrate (NO₃⁻) anions. To calculate its molecular mass, we need to sum the atomic masses of all the atoms present in one formula unit:
-
Identify the elements and their respective number: Calcium nitrate contains one calcium atom (Ca), two nitrogen atoms (N), and six oxygen atoms (O).
-
Find the atomic masses: Using the periodic table, we find the approximate atomic masses:
- Calcium (Ca): 40.08 amu
- Nitrogen (N): 14.01 amu
- Oxygen (O): 16.00 amu
-
Calculate the total mass:
- Mass of Ca: 1 Ca atom x 40.08 amu/Ca atom = 40.08 amu
- Mass of N: 2 N atoms x 14.01 amu/N atom = 28.02 amu
- Mass of O: 6 O atoms x 16.00 amu/O atom = 96.00 amu
-
Sum the individual masses:
- Total molecular mass = 40.08 amu + 28.02 amu + 96.00 amu = 164.10 amu
Therefore, the molecular mass of calcium nitrate is approximately 164.10 g/mol. Note that we can express this in either amu or g/mol, as they are numerically equivalent in this context.
Significance of Significant Figures
In scientific calculations, the accuracy of the result is limited by the least precise measurement. The atomic masses used are typically reported to two decimal places. Therefore, the final molecular mass should also be reported to two decimal places, ensuring consistency and reflecting the precision of the input data.
Applications of Molecular Mass Calculations
The calculation of molecular mass isn't just an academic exercise. It forms the basis for various crucial applications in chemistry and related fields:
1. Stoichiometry and Chemical Reactions
Knowing the molecular mass allows us to convert between mass and moles of a substance. This is fundamental for stoichiometric calculations, determining the quantities of reactants and products in a chemical reaction. For instance, if we want to determine how much calcium nitrate is needed to react with a specific amount of another compound, we must utilize its molecular mass for accurate conversion between grams and moles.
2. Solution Preparation
The molarity (M) of a solution, a crucial concentration unit, is defined as moles of solute per liter of solution. To prepare a solution of a specific molarity, we must first calculate the required mass of the solute using its molecular mass. For example, if you need to prepare 1 liter of 0.1 M calcium nitrate solution, you would use the molecular mass (164.10 g/mol) to calculate the grams of calcium nitrate required.
3. Determining Empirical and Molecular Formulas
Molecular mass plays a vital role in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms in a molecule. By comparing the empirical formula mass with the experimentally determined molecular mass, we can determine the molecular formula.
4. Gas Law Calculations
The ideal gas law (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). The molecular mass is essential for converting the mass of a gas to moles, allowing us to use the ideal gas law for various calculations involving gases.
5. Analytical Chemistry
In various analytical techniques like titration, gravimetric analysis, and spectrophotometry, the molecular mass is used for quantitative analysis. Accurate knowledge of the molecular mass ensures precise determination of the concentration or amount of a substance.
Beyond Calcium Nitrate: Extending the Calculation to Other Compounds
The method for calculating the molecular mass is applicable to all chemical compounds, both ionic and covalent. The process remains the same: identify the elements, obtain their atomic masses from the periodic table, and sum the weighted atomic masses to obtain the total molecular mass. For example, to calculate the molecular mass of sulfuric acid (H₂SO₄), you would follow the same steps, using the atomic masses of hydrogen, sulfur, and oxygen.
Dealing with Hydrates
Some compounds exist as hydrates, meaning they incorporate water molecules into their crystalline structure. For example, copper(II) sulfate pentahydrate (CuSO₄·5H₂O) contains five water molecules per formula unit. When calculating the molecular mass of a hydrate, remember to include the mass of the water molecules as well.
Isotopic Variations and Atomic Mass Precision
While we typically use the average atomic mass from the periodic table, it's important to note that variations exist due to the presence of different isotopes. For highly precise calculations, it might be necessary to consider the specific isotopic composition of the elements present in the compound.
Conclusion: Mastering Molecular Mass Calculations
Calculating the molecular mass of a compound like calcium nitrate is a fundamental skill in chemistry, impacting various applications ranging from stoichiometry to solution preparation and analytical techniques. By understanding the underlying principles, including atomic mass, Avogadro's number, and the significance of significant figures, one can confidently perform these calculations and utilize them effectively in different chemical contexts. Remember to always refer to an up-to-date periodic table for the most accurate atomic mass values. The accuracy of your calculations directly impacts the reliability of experimental results and theoretical predictions.
Latest Posts
Latest Posts
-
Is Carbon Metal Or Non Metal
Mar 26, 2025
-
What Is The Lcm For 6 And 12
Mar 26, 2025
-
What Is A Multiple Of 24
Mar 26, 2025
-
What Are The Common Factors Of 18 And 24
Mar 26, 2025
-
Difference Between Prokaryotic And Eukaryotic Transcription
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of Calcium Nitrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
