What Is The Molar Mass Of Ammonia Nh3
Juapaving
Apr 07, 2025 · 5 min read
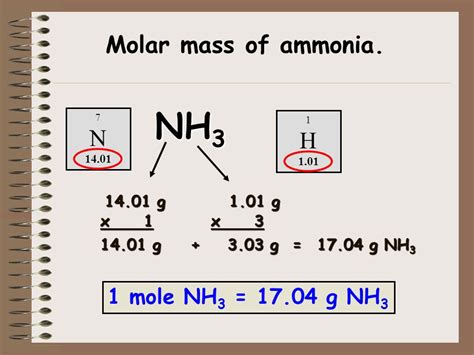
Table of Contents
What is the Molar Mass of Ammonia (NH₃)? A Comprehensive Guide
Ammonia, a ubiquitous compound with the chemical formula NH₃, plays a crucial role in various industrial processes and biological systems. Understanding its properties, particularly its molar mass, is fundamental to numerous chemical calculations and applications. This comprehensive guide delves deep into the concept of molar mass, specifically focusing on ammonia, providing clear explanations and practical examples.
Understanding Molar Mass
Before we calculate the molar mass of ammonia, let's establish a clear understanding of the term itself. Molar mass is defined as the mass of one mole of a substance. A mole, in chemistry, is a unit representing a specific number of particles – Avogadro's number, approximately 6.022 x 10²³. Therefore, the molar mass essentially tells us the mass of 6.022 x 10²³ molecules or formula units of a substance in grams. It's a critical conversion factor in stoichiometry, allowing us to relate the mass of a substance to the number of moles and vice versa.
The molar mass is expressed in grams per mole (g/mol). This unit allows for seamless integration into various chemical calculations, such as determining the amount of reactants needed for a specific reaction or calculating the yield of a product.
Calculating the Molar Mass of Ammonia (NH₃)
Ammonia (NH₃) is a simple molecule composed of one nitrogen atom (N) and three hydrogen atoms (H). To calculate its molar mass, we need to consider the atomic masses of its constituent elements. These atomic masses are typically found on the periodic table.
1. Identifying Atomic Masses:
- Nitrogen (N): The atomic mass of nitrogen is approximately 14.01 g/mol.
- Hydrogen (H): The atomic mass of hydrogen is approximately 1.01 g/mol.
2. Calculating the Molar Mass of NH₃:
The molar mass of NH₃ is the sum of the atomic masses of its constituent atoms, taking into account the number of each atom in the molecule:
Molar Mass (NH₃) = (1 x Atomic Mass of N) + (3 x Atomic Mass of H)
Molar Mass (NH₃) = (1 x 14.01 g/mol) + (3 x 1.01 g/mol)
Molar Mass (NH₃) = 14.01 g/mol + 3.03 g/mol
Molar Mass (NH₃) = 17.04 g/mol
Therefore, the molar mass of ammonia (NH₃) is approximately 17.04 grams per mole. This means that one mole of ammonia weighs approximately 17.04 grams.
Applications of Ammonia's Molar Mass
Knowing the molar mass of ammonia is crucial in various applications:
1. Stoichiometric Calculations: In chemical reactions involving ammonia, the molar mass acts as a conversion factor between mass and moles. This is essential for determining the amount of reactants needed or the expected yield of products. For instance, in the Haber-Bosch process (the industrial synthesis of ammonia), accurate molar mass calculations are crucial for optimizing the reaction's efficiency.
2. Solution Chemistry: When preparing ammonia solutions of specific concentrations (e.g., molarity), the molar mass is necessary for accurate calculations. Molarity is defined as moles of solute per liter of solution, and the molar mass allows for the conversion of mass to moles.
3. Gas Law Calculations: The ideal gas law (PV = nRT) uses the number of moles (n) to relate pressure (P), volume (V), temperature (T), and the ideal gas constant (R). Knowing the molar mass enables the calculation of the number of moles from the mass of ammonia gas, facilitating various gas law calculations.
4. Thermodynamic Calculations: In thermodynamic calculations involving ammonia, the molar mass is often required to determine properties such as enthalpy, entropy, and Gibbs free energy on a molar basis.
Importance of Accurate Atomic Masses
The accuracy of the molar mass calculation depends directly on the accuracy of the atomic masses used. While the values used above (14.01 g/mol for N and 1.01 g/mol for H) are approximations, more precise atomic masses can be obtained from authoritative sources such as the IUPAC (International Union of Pure and Applied Chemistry). Using more precise atomic masses will result in a more accurate molar mass for ammonia. For most general chemistry applications, however, the approximations used are sufficient.
Potential Sources of Error
While the calculation itself is straightforward, potential sources of error can arise:
- Rounding Errors: Rounding off atomic masses during calculations can introduce small errors. Using more significant figures in the atomic masses minimizes this error.
- Impurities: If the ammonia sample contains impurities, the calculated molar mass may differ from the theoretical value. High-purity ammonia is crucial for accurate results.
- Experimental Errors: If the molar mass is determined experimentally (e.g., using techniques like mass spectrometry), errors associated with the experimental method and instrumentation will affect the result.
Beyond the Basics: Isotopes and Molar Mass
The atomic masses used in the calculation are average atomic masses, reflecting the natural abundance of different isotopes of nitrogen and hydrogen. Isotopes are atoms of the same element with varying numbers of neutrons. For instance, nitrogen has two stable isotopes: ¹⁴N and ¹⁵N. The average atomic mass accounts for the relative abundances of these isotopes. If dealing with ammonia enriched in a specific isotope, the molar mass calculation would need to be adjusted accordingly using the specific isotopic mass.
Conclusion
The molar mass of ammonia (NH₃) is a fundamental property crucial in various chemical calculations and applications. Its precise determination hinges on accurate atomic masses and careful consideration of potential sources of error. Understanding this concept empowers chemists and scientists to accurately quantify and analyze reactions, solutions, and gases involving ammonia, solidifying its importance in both theoretical and practical chemistry. The value of approximately 17.04 g/mol provides a reliable basis for numerous calculations, underpinning our understanding of this vital compound. Remember to consult reliable sources for the most up-to-date and precise atomic mass values for the highest accuracy in your calculations.
Latest Posts
Latest Posts
-
The Three Medians Of A Triangle Intersect At The
Apr 08, 2025
-
What Is The Difference Between Cell Wall And Cell Membrane
Apr 08, 2025
-
How Many Hours In A Leap Year
Apr 08, 2025
-
What Is The Lcm Of 6 12 And 15
Apr 08, 2025
-
47 Inches Is How Many Feet
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Ammonia Nh3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.