What Is The Latent Heat Of Fusion
Juapaving
Mar 24, 2025 · 7 min read
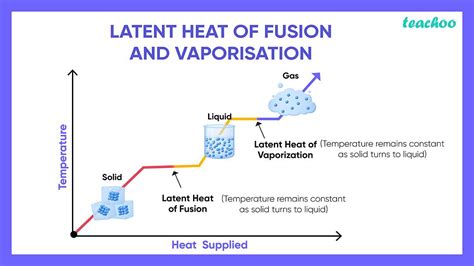
Table of Contents
What is the Latent Heat of Fusion? A Deep Dive into Phase Transitions
The world around us is constantly changing, and one of the most fundamental changes is the transition between different states of matter – solid, liquid, and gas. Understanding these transitions is crucial in various fields, from meteorology and material science to cooking and chemical engineering. Central to this understanding is the concept of latent heat, specifically the latent heat of fusion. This article delves deep into this fascinating phenomenon, exploring its definition, calculation, applications, and implications.
Defining Latent Heat of Fusion
Latent heat, in general, refers to the heat energy absorbed or released during a phase transition at a constant temperature. This means that the temperature of the substance doesn't change while it's undergoing the phase change; instead, the energy is used to break or form intermolecular bonds. The latent heat of fusion, also known as the enthalpy of fusion, specifically refers to the heat energy required to change one gram (or one mole) of a substance from a solid state to a liquid state at its melting point. Conversely, it's also the heat released when a substance changes from a liquid to a solid at its freezing point. This process occurs without any change in temperature.
Key Characteristics:
- Constant Temperature: The defining feature is that the temperature remains constant during the phase transition. All the energy goes into breaking the rigid structure of the solid.
- Phase Change: It's specifically the energy involved in changing from solid to liquid (melting) or liquid to solid (freezing).
- Substance Specific: The latent heat of fusion is a material property. Different substances have different values, reflecting the strength of their intermolecular forces. For example, water has a relatively high latent heat of fusion compared to many other substances.
Understanding the Molecular Perspective
To truly grasp the concept, let's explore it on a molecular level. In a solid, molecules are tightly packed in a regular, ordered structure. Strong intermolecular forces hold them in place. To melt the solid, enough energy must be supplied to overcome these forces and allow the molecules to move more freely, transitioning to the liquid phase. This energy is the latent heat of fusion. The molecules don't speed up (which would increase the temperature); instead, they gain the energy needed to break free from their fixed positions.
Think of it like this: Imagine a group of people tightly holding hands in a circle (solid). To break the circle (melt), each person needs enough energy to loosen their grip, allowing them to move freely (liquid). The energy required to break those bonds is analogous to the latent heat of fusion. Once the circle is broken, more energy can be added to make them move faster (increase temperature).
Calculating Latent Heat of Fusion
The latent heat of fusion (L<sub>f</sub>) can be calculated using the following formula:
Q = m * L<sub>f</sub>
Where:
- Q represents the heat energy (usually measured in Joules or calories).
- m represents the mass of the substance (usually in grams or kilograms).
- L<sub>f</sub> represents the latent heat of fusion (usually in J/g or cal/g).
This formula is particularly useful in practical applications. If you know the mass of a substance and the amount of heat energy added during melting, you can calculate the latent heat of fusion. Conversely, if you know the latent heat of fusion and the mass, you can calculate the energy required for melting.
Factors Affecting Latent Heat of Fusion
Several factors influence the value of the latent heat of fusion:
- Intermolecular Forces: Stronger intermolecular forces (e.g., hydrogen bonds in water) require more energy to break, resulting in a higher latent heat of fusion.
- Molecular Structure: The complexity and size of molecules influence the strength of interactions, affecting the latent heat of fusion.
- Pressure: Pressure can slightly affect the melting point and, consequently, the latent heat of fusion. However, this effect is usually minor for most substances.
- Impurities: The presence of impurities in a substance can alter its melting point and the latent heat of fusion.
Applications of Latent Heat of Fusion
The concept of latent heat of fusion finds applications in a wide variety of fields:
- Material Science: Understanding latent heat is crucial in material processing, such as casting metals, where controlled melting and solidification are essential.
- Meteorology: The latent heat released during the freezing of water plays a significant role in weather patterns, influencing cloud formation and temperature changes. The enormous amount of heat released when water freezes in the atmosphere can have significant impacts on weather systems.
- Food Science: The latent heat of fusion is essential in cooking and food preservation. The freezing of food relies on the release of latent heat.
- Chemical Engineering: In many industrial processes, the melting and solidification of materials require precise control, and understanding latent heat is vital for efficient operation.
- Climate Science: The melting and freezing of ice in polar regions plays a critical role in global climate change. Understanding the energy involved in these phase transitions is vital for climate modelling.
- Heating and Cooling Systems: The use of phase-change materials (PCMs) in thermal energy storage systems utilizes the latent heat of fusion to store and release thermal energy effectively.
Latent Heat of Fusion: Beyond the Basics
The discussion so far has primarily focused on the simple formula and its applications. However, a more nuanced understanding involves considering several advanced aspects:
Specific Heat Capacity and Latent Heat: A Combined Effect
While latent heat focuses on the energy during a phase change, specific heat capacity describes the energy required to change the temperature of a substance without a phase change. In real-world scenarios, both play a crucial role. For example, heating a block of ice to its melting point requires considering both the specific heat capacity of ice (to raise its temperature) and the latent heat of fusion (to melt it).
Latent Heat and Thermodynamics
Latent heat is intrinsically linked to thermodynamics. It relates directly to the change in enthalpy (heat content) during a phase transition. The enthalpy of fusion is a state function, meaning its value depends only on the initial and final states, not on the path taken.
Phase Diagrams and Latent Heat
Phase diagrams visually represent the relationship between temperature, pressure, and the phases of a substance. The latent heat of fusion is directly related to the slope of the solid-liquid equilibrium line on the phase diagram.
Determining Latent Heat Experimentally
While we can use the formula Q = m * L<sub>f</sub>, determining the latent heat of fusion often involves experimental methods such as calorimetry. These techniques accurately measure the heat absorbed or released during a phase transition, allowing for precise calculation of L<sub>f</sub>. Precise measurements are crucial for accurate modelling and predictions in scientific and engineering applications.
Examples of Latent Heat of Fusion Values
The latent heat of fusion varies widely depending on the substance. Here are a few examples:
- Water: Approximately 334 J/g (or 80 cal/g)
- Ice: Approximately 334 J/g (or 80 cal/g) - same as water due to the reversibility of the phase transition.
- Aluminum: Approximately 396 J/g
- Copper: Approximately 205 J/g
- Iron: Approximately 247 J/g
These values highlight the significant differences in the energy required to melt different substances.
Conclusion
The latent heat of fusion is a fundamental concept in physics and chemistry, with far-reaching implications across various disciplines. Understanding this concept is crucial for comprehending phase transitions, designing materials, predicting weather patterns, and many other applications. From the microscopic perspective of intermolecular forces to the macroscopic applications in industrial processes, latent heat of fusion is a crucial element in numerous scientific and engineering endeavors. This in-depth exploration has shed light on its definition, calculation, influencing factors, and wide-ranging applications, highlighting its importance in our understanding of the world around us.
Latest Posts
Latest Posts
-
Newtons Third Law Real Life Examples
Mar 26, 2025
-
What Is The Part Of Speech Of For
Mar 26, 2025
-
Whats The Square Root Of 125
Mar 26, 2025
-
What Is The Smallest Multiple Of 3 And 4
Mar 26, 2025
-
Steroid Hormones Exert Their Action By
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Latent Heat Of Fusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
