What Is The Freezing Point Of Water In Kelvin
Juapaving
Mar 21, 2025 · 5 min read
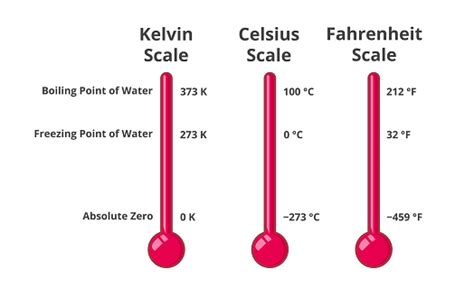
Table of Contents
What is the Freezing Point of Water in Kelvin? A Deep Dive into Temperature Scales and Their Significance
The freezing point of water is a fundamental concept in science, underpinning numerous applications across various fields. While most are familiar with 0° Celsius or 32° Fahrenheit, understanding its equivalent in Kelvin is crucial for scientific accuracy and precision. This article delves into the intricacies of the Kelvin scale, explains why it's the preferred scale for many scientific applications, and meticulously explores the freezing point of water within this context. We'll also touch upon related concepts like absolute zero and the importance of accurate temperature measurement.
Understanding Temperature Scales
Before we pinpoint the freezing point of water in Kelvin, let's establish a clear understanding of the different temperature scales commonly used. Three major scales dominate: Celsius, Fahrenheit, and Kelvin.
Celsius (°C)
The Celsius scale, also known as the centigrade scale, is widely used globally. It defines the freezing point of water as 0°C and the boiling point as 100°C at standard atmospheric pressure. Its simplicity and widespread adoption make it a convenient scale for everyday use.
Fahrenheit (°F)
Primarily used in the United States, the Fahrenheit scale sets the freezing point of water at 32°F and the boiling point at 212°F at standard atmospheric pressure. The scale's origins are historical and lack the inherent simplicity of the Celsius scale.
Kelvin (K)
The Kelvin scale, also known as the absolute temperature scale, stands apart from Celsius and Fahrenheit. Unlike the other two, it's not defined by the properties of water. Instead, it's based on absolute zero, the theoretical point at which all molecular motion ceases. This makes it particularly valuable in scientific contexts.
Key Differences: The crucial difference lies in the zero point. Celsius and Fahrenheit use arbitrary zero points based on water's properties, whereas Kelvin's zero point is absolute zero (0 K), approximately -273.15°C or -459.67°F. This absolute zero is a fundamental concept in thermodynamics.
The Freezing Point of Water in Kelvin: 273.15 K
The freezing point of water in Kelvin is 273.15 K. This value is not arbitrarily chosen; it's derived directly from the relationship between the Kelvin and Celsius scales:
K = °C + 273.15
Therefore, to convert the Celsius freezing point of water (0°C) to Kelvin, we simply add 273.15:
0°C + 273.15 = 273.15 K
This consistent and precise relationship allows for seamless transitions between the two scales in scientific calculations and analyses.
Why is the Kelvin Scale Preferred in Science?
The Kelvin scale's supremacy in scientific applications stems from its absolute nature. Several key advantages explain its prevalence:
-
Absolute Zero: The Kelvin scale's foundation in absolute zero provides a meaningful zero point. This allows for direct proportionality in many physical laws and equations. For example, the ideal gas law works most effectively using Kelvin temperatures.
-
Avoiding Negative Values: The absence of negative temperatures simplifies calculations and interpretations. This eliminates the complexities associated with negative temperatures in Celsius or Fahrenheit.
-
Thermodynamic Calculations: Many thermodynamic calculations, particularly involving energy and entropy, rely directly on Kelvin temperatures. The absolute nature of the scale simplifies these calculations considerably.
-
Consistency and Precision: The use of Kelvin ensures consistency and precision in scientific measurements and reporting. This is vital for replicating experiments and comparing results across different laboratories.
Applications Requiring Kelvin Scale Accuracy
The Kelvin scale's precision is essential across a wide array of scientific and engineering disciplines:
-
Physics: Areas like thermodynamics, statistical mechanics, and quantum mechanics rely heavily on Kelvin temperatures for accurate calculations and analysis.
-
Chemistry: Chemical reactions, phase transitions, and thermodynamic properties are often expressed and studied using the Kelvin scale.
-
Materials Science: Studying the behavior of materials at different temperatures, including phase transitions and material properties, requires precise Kelvin measurements.
-
Astronomy and Astrophysics: Understanding stellar temperatures, interstellar gas temperatures, and cosmological models necessitates the use of the Kelvin scale.
-
Meteorology: While Celsius is commonly used for weather reports, Kelvin plays a role in atmospheric modeling and understanding radiative transfer.
The Significance of Absolute Zero
Absolute zero (0 K) holds profound significance in physics. It represents the theoretical point at which all matter is devoid of kinetic energy. Atoms and molecules cease their vibrational and translational motion, reaching a state of minimum energy.
While absolute zero is theoretically unattainable, scientists have achieved remarkably low temperatures through cryogenics. These low-temperature experiments are crucial for understanding quantum phenomena and developing new technologies.
Practical Implications and Measurement
Accurately measuring temperature in Kelvin is critical for reliable scientific work. Various instruments are used, depending on the temperature range:
-
Thermocouples: These devices use the Seebeck effect to measure temperature differences. They are widely used across a broad range of temperatures.
-
Resistance Temperature Detectors (RTDs): These sensors rely on the change in electrical resistance of a material with temperature. They are known for their precision and stability.
-
Thermistors: These are semiconductor-based sensors with a high sensitivity to temperature changes. They are often used in applications requiring precise temperature control.
Beyond the Freezing Point: Water's Other Phase Transitions in Kelvin
The freezing point is just one aspect of water's behavior. Understanding its other phase transitions in Kelvin is equally important:
-
Boiling Point: The boiling point of water at standard atmospheric pressure is 373.15 K (100°C).
-
Triple Point: The triple point is the temperature and pressure at which water can exist in all three phases (solid, liquid, and gas) simultaneously. For water, it's approximately 273.16 K (0.01°C) and 611.657 Pa.
Conclusion: The Indispensable Kelvin Scale
The freezing point of water at 273.15 K underscores the Kelvin scale's significance in scientific research and engineering. Its absolute nature, avoidance of negative values, and direct proportionality in various physical laws make it the preferred scale for precise temperature measurements and calculations. Understanding the relationship between Kelvin and other temperature scales, as well as the concept of absolute zero, is crucial for anyone working in scientific fields or requiring high-precision temperature measurements. The widespread adoption and importance of the Kelvin scale highlight its enduring contribution to our understanding of the physical world. From everyday applications to groundbreaking research, the precise measurement and understanding of temperature remain fundamental to scientific progress.
Latest Posts
Latest Posts
-
Which Of These Are Components Of Dna Select Five Options
Mar 28, 2025
-
Why Does The Electronegativity Increase Across A Period
Mar 28, 2025
-
What Is The Smallest Unit Of Evolution
Mar 28, 2025
-
Least Common Multiple Of 14 And 49
Mar 28, 2025
-
Moment Of Inertia Of Quarter Circle
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
