What Is The Freezing Point Of Water In Fahrenheit
Juapaving
Mar 17, 2025 · 6 min read
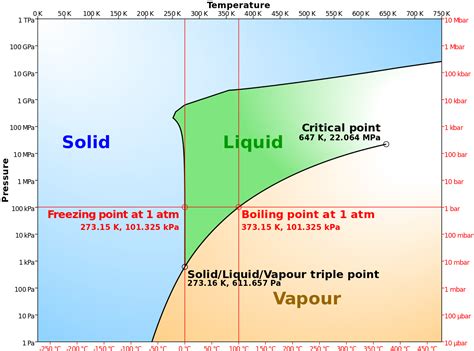
Table of Contents
What is the Freezing Point of Water in Fahrenheit? A Deep Dive into Water's Properties
The freezing point of water is a fundamental concept in science and everyday life. While many know it's 32° Fahrenheit (°F), understanding the nuances behind this seemingly simple number reveals a fascinating world of physics, chemistry, and even practical applications. This comprehensive guide delves into the freezing point of water in Fahrenheit, exploring its significance, influencing factors, and everyday relevance.
Understanding the Freezing Point
The freezing point of a substance is the temperature at which it transitions from a liquid to a solid state. For pure water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), this transition occurs at exactly 32° Fahrenheit (32°F). This is equivalent to 0° Celsius (°C) and 273.15 Kelvin (K). It's crucial to remember that this value is specific to pure water under standard conditions. Any deviation from these conditions – impurities, pressure changes – will impact the freezing point.
Why 32°F? A Look at the History of Temperature Scales
The seemingly arbitrary number, 32°F, stems from the historical development of the Fahrenheit scale. Developed by Daniel Gabriel Fahrenheit in the early 18th century, this scale initially used a zero point based on a mixture of ice, water, and ammonium chloride. Later, the scale was redefined, with 32°F representing the freezing point of water and 212°F representing its boiling point at standard atmospheric pressure. While the Celsius scale, with its more intuitive 0°C for freezing and 100°C for boiling, is now more widely used scientifically, the Fahrenheit scale remains prevalent in daily life in some parts of the world, particularly the United States.
Factors Affecting the Freezing Point of Water
While 32°F is the standard freezing point for pure water, several factors can influence this temperature:
1. Pressure: The Impact of Altitude
Pressure significantly impacts the freezing point of water. At higher altitudes, where atmospheric pressure is lower, water freezes at a slightly higher temperature than 32°F. This is because lower pressure reduces the energy required for the molecules to transition from the liquid to the solid phase. This effect is relatively small at altitudes typically experienced by humans, but it becomes more pronounced at extreme altitudes. This is why high-altitude cooking often requires adjustments to cooking times, as water boils at a lower temperature and freezes at a slightly higher temperature than at sea level.
2. Impurities: The Role of Dissolved Substances
The presence of dissolved substances, or solutes, in water lowers its freezing point. This phenomenon, known as freezing point depression, is a colligative property, meaning it depends on the concentration of solute particles, not their identity. The more solute particles present, the lower the freezing point. This is why saltwater freezes at a temperature below 32°F. This principle is utilized in various applications, such as de-icing roads in winter using salt or other chemicals that lower the freezing point of water on the road surfaces.
3. Supercooling: A Delayed Transition
Under specific conditions, water can remain in a liquid state even below its freezing point. This phenomenon is known as supercooling. It requires extremely pure water and the absence of nucleation sites – imperfections or impurities that provide a surface for ice crystals to form. Supercooled water is metastable; a slight disturbance, such as a vibration or the addition of a small ice crystal, will initiate rapid freezing.
4. Other factors
While less significant than pressure and impurities, other factors can subtly influence the freezing point of water including:
- Isotopic composition: The ratio of different isotopes of hydrogen and oxygen in the water molecule slightly affects the freezing point. Heavy water (D₂O), containing deuterium instead of hydrogen, freezes at a slightly higher temperature than regular water.
- Electric and magnetic fields: Extremely strong electric and magnetic fields can influence the freezing point of water. These effects are primarily of scientific interest, however, and not typically relevant in everyday situations.
The Significance of the Freezing Point of Water
The freezing point of water holds profound significance in numerous fields:
1. Environmental Sciences: Weather Patterns and Climate Change
The freezing and melting of water play a crucial role in weather patterns and climate change. Snow and ice formation, which are directly tied to the freezing point of water, influence global weather systems, ocean currents, and the Earth's climate. Changes in water's freezing point due to factors like rising temperatures and atmospheric composition significantly impact ecosystems and global climate models.
2. Biology: The Role of Water in Living Organisms
Water's unique properties, including its freezing point, are essential for life on Earth. The relatively high freezing point of water ensures that aquatic life can survive in freezing temperatures, as ice floats, creating an insulating layer on the water's surface. This layer protects aquatic organisms from freezing solid. The transition between liquid and solid water also plays vital roles in many biological processes.
3. Engineering and Technology: Applications in Various Industries
The freezing point of water is a critical factor in various engineering and technological applications. From the design of cooling systems and pipelines to the development of cryogenic technologies, a thorough understanding of water's freezing behavior is crucial. Industries from food processing and construction to transportation and energy rely heavily on manipulating the properties of water based on its freezing point. For example, understanding freeze-thaw cycles is critical for building materials and infrastructure design, ensuring structures can withstand temperature changes.
4. Everyday Life: From Winter Weather to Food Preservation
The freezing point of water significantly impacts our daily lives. Winter weather conditions, involving snow, ice, and freezing temperatures, directly relate to water's freezing point. The application of salt to de-ice roads and sidewalks utilizes the freezing point depression principle. Furthermore, freezing is a common method of food preservation, extending shelf life by inhibiting microbial growth. The freezing point of water is implicitly incorporated in numerous household appliances and everyday activities.
Measuring the Freezing Point of Water: Practical Considerations
Precisely measuring the freezing point of water requires careful attention to detail. The following factors are crucial:
- Purity of water: Using distilled or deionized water is essential to minimize the impact of impurities on the freezing point.
- Atmospheric pressure: The atmospheric pressure needs to be accurately measured and accounted for in the calculations. This is particularly critical at higher altitudes or in controlled laboratory settings.
- Calibration of the thermometer: The thermometer used must be accurately calibrated to ensure accurate temperature readings. Modern digital thermometers often provide higher accuracy and precision.
- Slow cooling rate: Allowing the water to cool gradually minimizes supercooling and ensures an accurate representation of the actual freezing point.
Conclusion: The Enduring Importance of 32°F
The freezing point of water at 32°F is more than just a number; it's a fundamental property with far-reaching implications. From shaping Earth's climate to enabling life as we know it, the transition of water between liquid and solid states underpins countless processes. Understanding this fundamental concept allows us to appreciate the intricate workings of nature and to develop technologies that benefit humanity. Its practical implications are widespread, impacting everything from infrastructure design to everyday activities, reinforcing its importance in various scientific and practical contexts. Future research into the nuances of water's freezing behavior, especially under non-standard conditions, will continue to enhance our understanding of this crucial aspect of our world.
Latest Posts
Latest Posts
-
Charles Darwin Was The First Person To Propose
Mar 17, 2025
-
Can A Magnet Ever Repel A Ferromagnetic Material
Mar 17, 2025
-
What Is The Charge Of Carbon Ion
Mar 17, 2025
-
How Many Valence Electrons Are In Sr
Mar 17, 2025
-
What Unit Of Measurement Is Used For Measuring Bacteria
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point Of Water In Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
