How Many Valence Electrons Are In Sr
Juapaving
Mar 17, 2025 · 5 min read
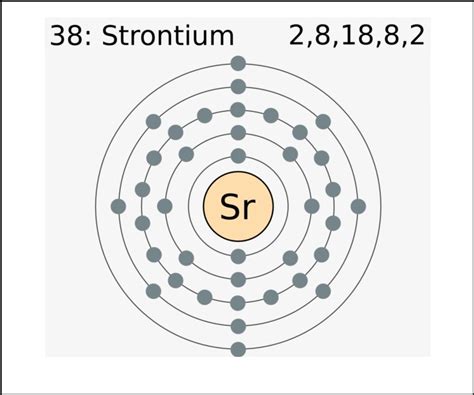
Table of Contents
How Many Valence Electrons Does Strontium (Sr) Have? A Deep Dive into Atomic Structure
Strontium (Sr), an alkaline earth metal, plays a fascinating role in various applications, from fireworks to medical imaging. Understanding its chemical behavior hinges on knowing its electronic configuration, particularly the number of valence electrons. This article delves deep into the atomic structure of strontium to determine its valence electron count, exploring related concepts like electron shells, orbitals, and periodic trends to provide a comprehensive understanding.
Understanding Valence Electrons: The Key to Chemical Reactivity
Valence electrons are the outermost electrons in an atom. These electrons are crucial because they are the ones involved in chemical bonding and interactions with other atoms. The number of valence electrons dictates an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). Atoms strive for stability, often achieved by gaining, losing, or sharing valence electrons to attain a full outer electron shell – a configuration similar to noble gases, which are exceptionally stable.
Electron Shells and Subshells: Building Blocks of Atomic Structure
Before we determine strontium's valence electrons, let's review the fundamental principles of atomic structure. Electrons are arranged in shells (also known as energy levels) around the nucleus. These shells are further divided into subshells (s, p, d, f), each capable of holding a specific number of electrons. The shells are numbered sequentially, starting with n=1 (closest to the nucleus), n=2, n=3, and so on. The subshells within a shell are filled according to the Aufbau principle (lowest energy levels filled first) and Hund's rule (electrons occupy orbitals singly before pairing up).
- Shell n=1: Holds a maximum of 2 electrons (1s subshell).
- Shell n=2: Holds a maximum of 8 electrons (2s and 2p subshells).
- Shell n=3: Holds a maximum of 18 electrons (3s, 3p, and 3d subshells).
- Shell n=4: Holds a maximum of 32 electrons (4s, 4p, 4d, and 4f subshells). And so on...
Determining the Valence Electrons of Strontium (Sr)
Strontium has an atomic number of 38, meaning it has 38 protons and 38 electrons in a neutral atom. To determine its valence electrons, we need to understand its electron configuration. This configuration shows how electrons are distributed among the various shells and subshells.
Strontium's Electron Configuration
The electron configuration of strontium is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s². This tells us:
- 1s²: Two electrons in the first shell (1s subshell).
- 2s²2p⁶: Eight electrons in the second shell (2s and 2p subshells).
- 3s²3p⁶3d¹⁰: Eighteen electrons in the third shell (3s, 3p, and 3d subshells).
- 4s²4p⁶: Eighteen electrons in the fourth shell (4s and 4p subshells).
- 5s²: Two electrons in the fifth shell (5s subshell).
Identifying the Valence Electrons
The valence electrons are the electrons in the outermost shell. In strontium's case, the outermost shell is the fifth shell (n=5), which contains two electrons in the 5s subshell. Therefore, strontium has two valence electrons.
Strontium's Position in the Periodic Table and its Valence Electrons
Strontium's position in the periodic table further confirms its valence electron count. It belongs to Group 2, also known as the alkaline earth metals. Elements within this group are characterized by having two valence electrons in their outermost shell. This commonality explains the similar chemical properties observed within the group, such as their reactivity with water and oxygen.
Chemical Behavior and Bonding of Strontium: The Role of Valence Electrons
The presence of two valence electrons significantly influences strontium's chemical behavior. To achieve a stable noble gas configuration (like krypton), strontium readily loses its two valence electrons, forming a +2 cation (Sr²⁺). This is a characteristic feature of alkaline earth metals. This electron loss is the driving force behind strontium's reactivity.
Ionic Bonding in Strontium Compounds
Strontium typically forms ionic bonds with nonmetals. For example, when strontium reacts with chlorine (Cl), it loses its two valence electrons to form Sr²⁺ ions, while chlorine atoms gain one electron each to form Cl⁻ ions. The electrostatic attraction between the oppositely charged ions results in the formation of strontium chloride (SrCl₂), a stable ionic compound.
Metallic Bonding in Strontium Metal
Strontium, in its elemental form, exhibits metallic bonding. In metallic bonding, valence electrons are delocalized and form a "sea" of electrons surrounding positively charged metal ions. This electron sea allows for good electrical and thermal conductivity, characteristic of metals.
Applications of Strontium: A Consequence of its Electronic Structure
The unique electronic structure of strontium, specifically its two valence electrons and resulting +2 oxidation state, underpins its use in various applications:
-
Pyrotechnics: Strontium salts impart a brilliant red color to fireworks due to the excitation and subsequent relaxation of its electrons.
-
Medical Applications: Strontium-89 is a radioactive isotope used in the treatment of bone cancer. Its chemical behavior, similar to calcium, allows it to target bone tissue.
-
Alloys: Strontium is used in some aluminum alloys to improve their castability and mechanical properties.
Conclusion: Understanding Valence Electrons is Key
The number of valence electrons is a critical aspect of understanding an element's chemical behavior. Strontium, with its two valence electrons, exemplifies the importance of this concept. Its position in the periodic table, electron configuration, and resulting chemical reactivity are all directly related to its electron arrangement. This understanding is fundamental to comprehending its diverse applications and its role in various scientific and industrial processes. The consistent presence of two valence electrons in strontium is a defining characteristic of alkaline earth metals, and it plays a crucial role in shaping its chemical and physical properties, making strontium a fascinating element to study. Further exploration into its isotopic variations and their specific applications can provide a deeper appreciation for the impact of this element on various fields of science and technology.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Tropic Hormone
Mar 17, 2025
-
Write 100 As A Product Of Prime Factors
Mar 17, 2025
-
What Is The Advantage Of Having Four Chambered Heart
Mar 17, 2025
-
An Area Of Land That Is Completely Surrounded By Water
Mar 17, 2025
-
Lcm Of 5 6 And 3
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Sr . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
