What Is The Charge Of Carbon Ion
Juapaving
Mar 17, 2025 · 6 min read
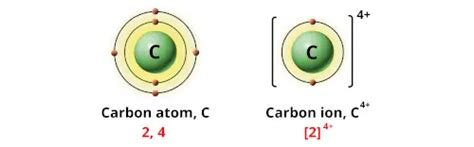
Table of Contents
What is the Charge of a Carbon Ion? Understanding Carbon's Ionic States
The charge of a carbon ion isn't a single, fixed value. Unlike some elements that consistently exhibit one specific ionic charge, carbon's ionic state is highly variable depending on its chemical environment and bonding interactions. This article delves into the complexities of carbon's ionization, exploring the different ionic charges it can adopt, the factors influencing these charges, and the implications for its chemical behavior.
Understanding Ionization: The Basics
Before we delve into the specifics of carbon ions, let's establish a fundamental understanding of ionization. Ionization is the process by which an atom or molecule acquires a net electrical charge by gaining or losing electrons. When an atom loses electrons, it becomes a cation, carrying a positive charge. When an atom gains electrons, it becomes an anion, carrying a negative charge. The magnitude of the charge depends on the number of electrons gained or lost.
Carbon's Electronic Structure: The Foundation of Ionization
Carbon's position in the periodic table – Group 14 – dictates its electron configuration. A neutral carbon atom has six electrons: two in the first shell (1s²) and four in the second shell (2s²2p²). These four electrons in the outer shell are valence electrons, meaning they participate in chemical bonding. This arrangement gives carbon its remarkable versatility in forming various compounds.
Common Carbon Ions and Their Charges
While carbon readily forms covalent bonds (sharing electrons), it can also exist as ions, though this is less common than covalent bonding. The most common ionic charges for carbon are:
+4 (C⁴⁺): The Tetrapositive Carbon Ion
This is a highly unstable ion, rarely encountered in typical chemical scenarios. The removal of four electrons from carbon requires a substantial amount of energy, making it energetically unfavorable under most conditions. The high positive charge density makes it extremely reactive, readily attracting electrons from its surroundings. This state might be encountered in extremely high-energy environments or theoretical calculations.
+2 (C²⁺): The Dipositive Carbon Ion
Slightly more stable than the +4 ion, the +2 ion is still relatively uncommon in standard chemical contexts. The removal of two electrons requires considerable energy, and the resulting ion is highly reactive. The +2 ion is more likely to be observed in specific high-energy processes or as an intermediate species in complex reactions.
-4 (C⁴⁻): The Tetranegative Carbon Ion
This is arguably the more "stable" of the carbon ions, although "stable" in this context is relative. The addition of four electrons to the carbon atom's outer shell completes its octet, conforming to the octet rule (although the octet rule is not universally applicable). However, the high negative charge density makes it highly reactive, readily attracting positively charged species. The tetranegative carbon ion is encountered in certain carbide compounds (such as metal carbides) where the high electronegativity of the metal compensates for the negative charge on the carbon.
Other Less Common Ionic States
While the +4, +2, and -4 charges are the most frequently discussed, carbon can theoretically exist in other ionic states under highly specialized conditions. For example, under extreme conditions such as those found in stellar atmospheres or plasma physics, carbon ions with charges of +3 or +1 might be observed. The stability and prevalence of these less common ionic states depend heavily on the specific environmental factors.
Factors Influencing Carbon's Ionic State
Several factors determine which ionic state carbon adopts, including:
Electronegativity of the Bonding Partner
When carbon forms bonds with other atoms, the electronegativity difference between carbon and its partner significantly impacts the charge distribution. When bonding with highly electronegative atoms (such as oxygen or fluorine), carbon can exhibit a partial positive charge as electrons are drawn towards the more electronegative partner. Conversely, when bonding with less electronegative atoms (such as metals), carbon can exhibit a partial negative charge.
Oxidation State
The oxidation state of carbon represents the hypothetical charge on carbon if all bonds were completely ionic. This provides a convenient way to track electron transfer in chemical reactions. The oxidation state can vary from -4 (in methane, CH₄) to +4 (in carbon dioxide, CO₂), illustrating the range of possible charge distributions.
Chemical Environment
The surrounding chemical environment plays a crucial role in influencing carbon's ionic state. Factors such as solvent polarity, temperature, and pressure can affect the stability and likelihood of different ionic states.
Type of Bond
The type of bond formed – ionic, covalent, or polar covalent – significantly influences the apparent charge on carbon. In purely ionic bonds, the charge is formally assigned. In covalent bonds, the charge is distributed across the bond, leading to partial charges.
Implications of Carbon's Variable Ionic State
The variable ionic state of carbon profoundly affects its chemical properties and its role in various chemical processes. The different ionic charges influence:
Reactivity:
Highly charged carbon ions (both positive and negative) are extremely reactive due to their high charge density. They readily participate in redox reactions (reduction-oxidation reactions involving electron transfer).
Bonding:
The charge on carbon affects the type of bonds it forms and the nature of the resulting molecules. Positive carbon ions tend to form bonds with negatively charged species, while negative carbon ions interact with positively charged species.
Spectroscopic Properties:
The ionic state of carbon significantly alters its spectroscopic properties, including its absorption and emission spectra. These changes can be used to identify and characterize different carbon species in various environments.
Carbon's Importance in Biological and Industrial Settings
Carbon's ability to form a wide variety of stable compounds underpins its central role in organic chemistry and biochemistry. Its versatility in forming various ionic states, though less prevalent than its covalent bonding tendencies, still influences its behavior in crucial chemical processes. In industrial settings, understanding carbon's ionic properties is important in materials science, particularly in the development of new carbon-based materials with tailored properties.
Conclusion: The Dynamic Nature of Carbon Ions
The charge of a carbon ion is not a simple, fixed value. It is highly context-dependent and variable, ranging from +4 to -4, depending on factors like its chemical environment, bonding partners, and oxidation state. While the fully charged ions (+4 and -4) are rarely observed under normal conditions, understanding their theoretical existence and the factors influencing partial charges contributes to a complete picture of carbon's behavior in various chemical systems. The dynamic nature of carbon's ionic states underscores its remarkable chemical versatility and pivotal role in both the natural world and industrial applications. Further research into the less-common ionic states and their behavior under extreme conditions holds potential for scientific advancements across numerous disciplines.
Latest Posts
Latest Posts
-
Does A Jellyfish Have Radial Symmetry
Mar 17, 2025
-
How Many Sperm Cells Form From A Primary Spermatocyte
Mar 17, 2025
-
How Do You Spell 60 In Words
Mar 17, 2025
-
Which Blood Vessel Will Have The Greatest Amount Of Oxygen
Mar 17, 2025
-
Select All The Characteristics Of Bryophytes
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Carbon Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
