What Is The Formula For The Compound Iron Iii Sulfate
Juapaving
Mar 17, 2025 · 6 min read
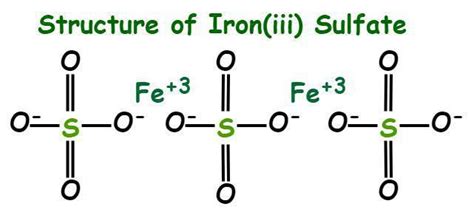
Table of Contents
Decoding the Formula for Iron(III) Sulfate: A Deep Dive into Chemistry
Iron(III) sulfate, also known as ferric sulfate, is a fascinating chemical compound with a wide array of applications. Understanding its chemical formula is key to comprehending its properties and uses. This article will delve deep into the formula, explaining its components, its different forms, and its significance in various fields. We'll also explore related concepts such as oxidation states, chemical nomenclature, and the importance of accurate formula representation.
Understanding Chemical Formulas: A Primer
Before we jump into the specifics of iron(III) sulfate, let's establish a basic understanding of chemical formulas. A chemical formula is a concise way of representing the composition of a chemical compound using chemical symbols and numbers. These symbols represent the elements present, while the numbers (subscripts) indicate the relative number of atoms of each element in the molecule.
For example, the formula for water, H₂O, tells us that each water molecule contains two hydrogen atoms (H) and one oxygen atom (O). The subscripts are crucial; changing them alters the compound entirely.
Unveiling the Formula: Fe₂(SO₄)₃
The chemical formula for iron(III) sulfate is Fe₂(SO₄)₃. Let's break it down:
- Fe: This represents the element iron (Ferrum).
- 2: This subscript indicates that there are two iron atoms in each molecule of iron(III) sulfate.
- (SO₄): This is the sulfate ion, a polyatomic ion composed of one sulfur atom (S) and four oxygen atoms (O). The parentheses indicate that this entire group acts as a single unit.
- 3: This subscript indicates that there are three sulfate ions associated with the two iron atoms.
Therefore, the formula Fe₂(SO₄)₃ signifies that one molecule of iron(III) sulfate contains two iron atoms and three sulfate ions, resulting in a total of two iron atoms, three sulfur atoms, and twelve oxygen atoms (3 x 4 = 12).
The Significance of the Roman Numeral (III)
The Roman numeral III in the name "iron(III) sulfate" is crucial. It indicates the oxidation state of the iron atom. Iron is a transition metal, meaning it can exhibit multiple oxidation states. The oxidation state represents the charge an atom would have if all its bonds were completely ionic.
In iron(III) sulfate, the iron atom has an oxidation state of +3. This means it has lost three electrons. The sulfate ion (SO₄²⁻) carries a charge of -2. To achieve charge neutrality in the compound, two iron(III) ions (+3 each) are needed to balance the charge of three sulfate ions (-2 each). This is why the formula has two iron atoms and three sulfate ions.
Contrast this with iron(II) sulfate (ferrous sulfate), which has the formula FeSO₄. Here, the iron atom has an oxidation state of +2.
Different Forms and Hydrates of Iron(III) Sulfate
Iron(III) sulfate doesn't exist solely as the anhydrous form represented by Fe₂(SO₄)₃. It often occurs as hydrates, meaning water molecules are incorporated into its crystal structure. Common hydrates include:
- Iron(III) sulfate heptahydrate (Fe₂(SO₄)₃·7H₂O): This is perhaps the most common form, containing seven water molecules per formula unit.
- Iron(III) sulfate nonahydrate (Fe₂(SO₄)₃·9H₂O): This form contains nine water molecules per formula unit.
The presence of water molecules affects the compound's properties, such as solubility and color. The anhydrous form is typically a pale yellow-brown, while the hydrates are often yellowish or brownish-white.
Applications of Iron(III) Sulfate: A Diverse Range
The versatile nature of iron(III) sulfate makes it useful in many applications:
- Water Treatment: Iron(III) sulfate is widely used as a coagulant in water treatment plants. It helps to flocculate suspended particles, making them easier to remove.
- Wastewater Treatment: It plays a role in removing phosphorus from wastewater, reducing its environmental impact.
- Agriculture: It serves as a soil amendment, providing iron to plants, and is also used as a herbicide and algaecide.
- Pigments and Dyes: Historically, it has been used in the production of pigments and dyes, although its use in this area has diminished due to the development of more stable and less toxic alternatives.
- Medicine (Historically): In the past, it found limited use in medicine as an astringent.
- Textile Industry: It has been utilized in textile processing for various purposes.
- Catalysis: It acts as a catalyst in some chemical reactions.
Safety Considerations: Handling Iron(III) Sulfate
Like any chemical compound, iron(III) sulfate requires careful handling. It is an irritant and can cause skin and eye irritation. Inhalation of dust can be harmful. Therefore, always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a respirator, when handling iron(III) sulfate. Proper ventilation is also essential to avoid inhaling dust or fumes.
Related Chemical Concepts
Understanding the formula for iron(III) sulfate necessitates familiarity with several related chemical concepts:
- Ionic Compounds: Iron(III) sulfate is an ionic compound, meaning it is formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions).
- Polyatomic Ions: The sulfate ion (SO₄²⁻) is a polyatomic ion, a group of atoms that carries an overall charge.
- Oxidation States: As mentioned earlier, understanding oxidation states is crucial for determining the correct chemical formula.
- Chemical Nomenclature: The systematic naming of chemical compounds follows specific rules; learning these rules is essential for correctly naming and identifying chemical substances.
- Stoichiometry: Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Knowing the formula of iron(III) sulfate is fundamental for performing stoichiometric calculations involving this compound.
- Hydration: The ability of salts to incorporate water molecules into their crystal structures.
Further Exploration: Beyond the Basics
While this article provides a comprehensive overview of the formula and properties of iron(III) sulfate, further exploration can be undertaken to deepen your understanding:
- Spectroscopic analysis: Techniques such as UV-Vis spectroscopy can be used to characterize iron(III) sulfate solutions.
- Crystallography: X-ray crystallography can reveal the precise arrangement of atoms within the crystal lattice of iron(III) sulfate hydrates.
- Thermodynamic properties: Studying the thermodynamic properties such as enthalpy and entropy can provide insights into the stability and reactivity of iron(III) sulfate.
- Chemical reactions: Investigating the reactions iron(III) sulfate undergoes with other substances can reveal its chemical behavior.
In conclusion, the seemingly simple formula Fe₂(SO₄)₃ for iron(III) sulfate encapsulates a wealth of chemical information. Understanding this formula, along with the related concepts discussed, unlocks a deeper comprehension of this important compound and its diverse applications in various fields. Remember always to prioritize safety when handling chemicals and to consult relevant safety data sheets (SDS) before working with any chemical substance.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For The Compound Iron Iii Sulfate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
