What Is The Formula For Nitride Ion
Juapaving
Mar 21, 2025 · 5 min read
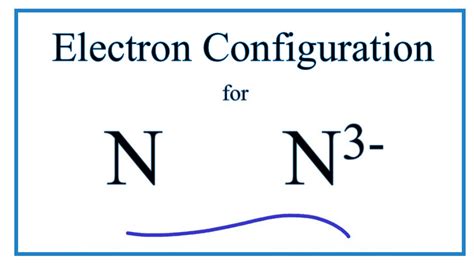
Table of Contents
What is the Formula for Nitride Ion? A Deep Dive into Nitrogen Chemistry
Nitrogen, a cornerstone element in the periodic table, exhibits fascinating chemical behavior, forming a diverse range of compounds. Among these, nitride ions hold a unique position, playing a crucial role in various materials and chemical processes. This article will delve deep into the nitride ion, exploring its formula, formation, properties, and applications. We'll also touch upon related concepts to provide a comprehensive understanding of this important chemical species.
Understanding the Nitride Ion: Formula and Formation
The nitride ion is represented by the chemical formula N³⁻. This signifies a nitrogen atom that has gained three electrons, achieving a stable octet configuration similar to that of the noble gas neon. The negative three charge indicates the presence of three extra electrons, resulting in a significant ionic character.
The Octet Rule and Nitride Ion Stability
The formation of the nitride ion is a prime example of the octet rule in action. Nitrogen, with five valence electrons, needs three more to complete its outer electron shell. By gaining these three electrons, it achieves a stable electron configuration, making the nitride ion relatively stable. This stability is crucial for its participation in various chemical reactions and the formation of stable nitride compounds.
Formation Mechanisms: Reactions Leading to Nitride Ions
The formation of nitride ions usually involves the reaction of elemental nitrogen (N₂) with highly reactive metals or at extremely high temperatures. Nitrogen gas, with its strong triple bond, is quite inert under normal conditions. However, under specific circumstances, it can react:
-
Reactions with Alkali and Alkaline Earth Metals: These highly electropositive metals readily donate electrons to nitrogen, forming nitrides. For example, the reaction of lithium with nitrogen produces lithium nitride: 6Li + N₂ → 2Li₃N
-
High-Temperature Reactions: At very high temperatures, nitrogen can react with other elements, even those less reactive than alkali metals. This often requires specialized conditions, such as plasma or specialized furnaces. For example, the formation of boron nitride (BN) requires high temperatures: 2B + N₂ → 2BN
-
Reactions in Liquid Ammonia: In certain specialized reactions within liquid ammonia, the reduction of nitrogen can lead to nitride formation, offering an alternative pathway to this negatively charged ion.
Properties of Nitride Ions and Nitride Compounds
Nitride ions, being highly charged anions, significantly influence the properties of the compounds they form. These properties vary widely depending on the cation they are bonded with:
Ionic Character and Crystal Structure
Nitride compounds generally exhibit ionic character, especially those formed with alkali and alkaline earth metals. The strong electrostatic attraction between the highly charged nitride ion (N³⁻) and the positively charged metal cations leads to the formation of crystalline structures. The precise crystal structure depends on the size and charge of the metal cation, resulting in a variety of structures such as cubic, hexagonal, or complex layered arrangements.
Hardness and Refractoriness
Many nitride compounds, such as boron nitride (BN) and silicon nitride (Si₃N₄), are known for their exceptional hardness and high melting points (refractoriness). These properties make them suitable for applications where high-temperature stability and resistance to wear and tear are crucial.
Electrical Conductivity
The electrical conductivity of nitride compounds varies depending on their chemical composition and crystal structure. Some nitride compounds can exhibit semiconducting or even metallic behavior. This is partly determined by the availability of electrons and the band structure of the solid.
Chemical Reactivity
Nitride compounds demonstrate diverse chemical reactivity, influenced by the specific metal cation. Some nitrides are relatively stable in air and water, while others readily react with acids or water, producing ammonia (NH₃).
Applications of Nitride Compounds: From LEDs to Cutting Tools
The unique properties of nitride compounds make them indispensable in a wide range of applications:
Light-Emitting Diodes (LEDs)
Gallium nitride (GaN) and related compounds are crucial components in high-brightness LEDs. Their ability to emit light across a wide range of wavelengths, from ultraviolet to visible, makes them essential in various applications, including lighting, displays, and optical communications.
Cutting Tools and Abrasives
The exceptional hardness of materials like cubic boron nitride (c-BN) and silicon nitride (Si₃N₄) make them ideal for use in cutting tools and abrasives. Their ability to withstand high temperatures and pressures during machining makes them superior to traditional materials in many applications.
Refractory Materials
Nitride ceramics, such as silicon nitride and boron nitride, exhibit excellent high-temperature stability, making them suitable for applications in high-temperature environments, such as furnace linings and engine components.
Electronic Applications
Certain nitrides find applications in the electronics industry due to their semiconducting properties or their ability to form insulating layers. They are utilized in transistors, integrated circuits, and other microelectronic devices.
Coatings and Protective Layers
Nitride coatings are frequently used to enhance the surface properties of other materials. They can provide improved hardness, wear resistance, corrosion resistance, and thermal stability.
Catalysts
Some nitride compounds act as catalysts in various chemical reactions, increasing reaction rates and improving efficiency. Their catalytic properties often depend on their surface area and electronic structure.
Related Concepts and Further Exploration
To gain a more comprehensive understanding of nitride ions, it's helpful to explore related concepts:
-
Nitriding: This process involves introducing nitrogen into the surface of a metal to enhance its hardness and wear resistance. It is a common surface modification technique used in various industries.
-
Azides: These compounds contain the azide ion (N₃⁻), which is related to but distinct from the nitride ion (N³⁻). Azides are often explosive and highly reactive.
-
Nitriles: These organic compounds contain the cyano group (-CN), a functional group with a carbon-nitrogen triple bond. Nitriles are common in organic chemistry and have various applications.
Conclusion: The Nitride Ion - A Versatile Chemical Species
The nitride ion (N³⁻), with its unique properties and reactivity, plays a significant role in various aspects of chemistry and materials science. From the vibrant colors of LEDs to the durability of cutting tools, nitride compounds are indispensable in a wide array of modern technologies. The ongoing research and development in this area promise further advancements and exciting applications in the future. The continued study of nitride ion formation, properties, and applications will undoubtedly contribute to innovation in materials science, electronics, and other fields. Understanding the fundamental chemistry behind this seemingly simple ion opens doors to a wide array of possibilities.
Latest Posts
Latest Posts
-
Rectangular Form To Polar Form Converter
Mar 28, 2025
-
What Are The Factors Of 102
Mar 28, 2025
-
Why Do We Not Have Lunar Eclipses Every Month
Mar 28, 2025
-
Whats The Difference Between Monohybrid And Dihybrid Crosses
Mar 28, 2025
-
Least Common Multiple Of 12 15
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For Nitride Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
