What Is The Formula For Iron Iii Sulfate
Juapaving
Mar 17, 2025 · 5 min read
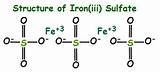
Table of Contents
What is the Formula for Iron(III) Sulfate? A Deep Dive into its Chemistry and Applications
Iron(III) sulfate, also known as ferric sulfate, is an inorganic compound with a rich history and a wide range of applications. Understanding its chemical formula is crucial to grasping its properties and uses. This comprehensive guide delves into the formula, structure, properties, production methods, and diverse applications of this important chemical.
Understanding the Chemical Formula: Fe₂(SO₄)₃
The chemical formula for iron(III) sulfate is Fe₂(SO₄)₃. Let's break this down:
-
Fe: Represents the element iron (Ferrum). The Roman numeral III indicates that iron is in its +3 oxidation state. This is crucial, as iron can exist in multiple oxidation states (+2 and +3 being the most common). Iron(II) sulfate, or ferrous sulfate, has a different formula and properties.
-
SO₄: Represents the sulfate anion (SO₄²⁻), a polyatomic ion consisting of one sulfur atom and four oxygen atoms. It carries a -2 charge.
-
₂ and ₃: These subscripts indicate the number of each ion needed to balance the charges. Two iron(III) ions (each with a +3 charge, totaling +6) are needed to balance three sulfate ions (each with a -2 charge, totaling -6). The overall charge of the compound is neutral.
The Structure of Iron(III) Sulfate
The structure of iron(III) sulfate is more complex than simply a linear arrangement of ions. It exists in several forms, depending on the hydration state (the number of water molecules bound to the compound). The most common forms are:
-
Anhydrous Iron(III) Sulfate: Fe₂(SO₄)₃ without any water molecules. This form is a pale yellow to white powder.
-
Hydrated Iron(III) Sulfate: Various hydrates exist, with the most common being Iron(III) sulfate nonahydrate, Fe₂(SO₄)₃·9H₂O. This is a pale yellow or yellowish-brown crystalline solid. The water molecules are incorporated into the crystal lattice, influencing the physical properties like solubility and color.
Physical and Chemical Properties of Iron(III) Sulfate
The properties of iron(III) sulfate vary depending on the hydration state. However, some general properties include:
-
Appearance: Anhydrous form is a pale yellow or white powder, while hydrates are pale yellow or yellowish-brown crystals.
-
Solubility: Soluble in water, forming acidic solutions. The solubility increases with temperature.
-
Melting Point: Decomposes upon heating before melting, releasing sulfur trioxide (SO₃) and eventually forming iron(III) oxide (Fe₂O₃).
-
Density: Varies depending on the hydrate.
-
Acidity: Aqueous solutions are acidic due to the hydrolysis of the iron(III) ion, leading to the release of H⁺ ions.
-
Reactivity: Reacts with various substances, including bases, reducing agents, and other metals.
Production of Iron(III) Sulfate
Iron(III) sulfate can be produced through several methods:
-
Reaction of Iron(III) Oxide with Sulfuric Acid: This is the most common industrial method. Iron(III) oxide (Fe₂O₃), often obtained from iron ore processing, is reacted with sulfuric acid (H₂SO₄):
Fe₂O₃ + 3H₂SO₄ → Fe₂(SO₄)₃ + 3H₂O
-
Oxidation of Iron(II) Sulfate: Iron(II) sulfate (FeSO₄) can be oxidized using oxidizing agents like hydrogen peroxide (H₂O₂) or nitric acid (HNO₃) to produce iron(III) sulfate.
-
Reaction of Iron with Sulfuric Acid: Iron metal can react with concentrated sulfuric acid to produce iron(III) sulfate, but this reaction is less commonly used for large-scale production.
The specific method chosen depends on factors like cost, availability of raw materials, and desired purity.
Applications of Iron(III) Sulfate
Iron(III) sulfate's versatile properties lead to a wide array of applications across various industries:
1. Water Treatment:
-
Coagulation and Flocculation: Iron(III) sulfate is a crucial coagulant used in wastewater treatment plants. It neutralizes negatively charged particles in water, causing them to clump together and settle out, improving water clarity.
-
Removal of Phosphate: It is effective in removing excess phosphates from wastewater, preventing eutrophication (excessive nutrient enrichment) in receiving water bodies.
-
Iron Removal: Paradoxically, it can be used to remove dissolved iron from water, although careful control is needed to avoid introducing more iron.
2. Agriculture:
-
Soil Amendment: Iron(III) sulfate is used as a soil amendment to correct iron deficiency in plants, particularly in alkaline soils where iron availability is limited. It provides a readily available source of iron for plant uptake.
-
Herbicide and Fungicide: In certain applications, it can act as a herbicide and fungicide.
-
Coloring Agent: It is sometimes used as a coloring agent in fertilizers to enhance their visual appeal.
3. Industrial Applications:
-
Dyeing and Printing: It plays a role as a mordant in dyeing fabrics, helping dyes bind to the fibers more effectively.
-
Pigment Production: Used in the production of iron oxide pigments, which are used in paints, plastics, and other materials.
-
Etching: It is involved in etching processes for various materials.
-
Catalyst: In some chemical processes, it acts as a catalyst.
4. Medical Applications:
While less common than its industrial uses, iron(III) sulfate has some medical applications:
- Treatment of Iron Deficiency Anemia: It can be a source of iron in treating iron deficiency anemia (though other forms of iron are more commonly used).
Environmental Considerations
While iron(III) sulfate has many benefits, its environmental impact must be considered. Improper disposal can lead to water contamination and affect aquatic life. Sustainable practices, responsible handling, and appropriate waste management are essential in minimizing environmental risks.
Safety Precautions
Iron(III) sulfate is considered a relatively low-hazard chemical compared to many other industrial chemicals. However, precautions should still be taken:
-
Eye and Skin Protection: Always wear appropriate eye and skin protection when handling iron(III) sulfate.
-
Inhalation: Avoid inhalation of dust, as it can irritate the respiratory system.
-
Ingestion: Do not ingest iron(III) sulfate.
-
Storage: Store in a cool, dry place, away from incompatible materials.
Conclusion
Iron(III) sulfate, with its chemical formula Fe₂(SO₄)₃, is a versatile inorganic compound with significant applications in various fields. Understanding its properties, production, and uses is crucial for its safe and effective application. While generally considered safe, responsible handling and environmentally conscious practices are essential to mitigate any potential risks. This multifaceted compound continues to play a vital role in diverse industrial processes and contributes significantly to human advancements. Further research and innovation promise to expand its applications even further in the future.
Latest Posts
Latest Posts
-
What Is The Boiling Point For Kelvin
Mar 17, 2025
-
What Is The Least Common Multiple Of 14 And 12
Mar 17, 2025
-
Plants That Make Their Own Food Are Called
Mar 17, 2025
-
What Is The Lcm Of 11 And 3
Mar 17, 2025
-
What Is 3 100 As A Decimal
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For Iron Iii Sulfate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
