What Is The Boiling Point For Kelvin
Juapaving
Mar 17, 2025 · 5 min read
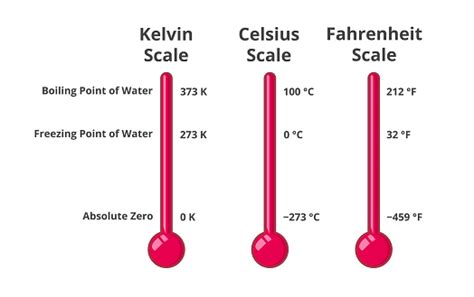
Table of Contents
What is the Boiling Point in Kelvin? Understanding Temperature Scales and Phase Transitions
The boiling point of a substance, the temperature at which it transitions from a liquid to a gas, is a crucial physical property. Understanding this property, particularly when expressed in Kelvin, is fundamental in various scientific and engineering disciplines. This comprehensive article delves into the concept of boiling point, its relationship to Kelvin, and explores its importance across different applications.
Understanding Temperature Scales: Celsius, Fahrenheit, and Kelvin
Before diving into the specifics of boiling points in Kelvin, let's briefly review the three most common temperature scales: Celsius (°C), Fahrenheit (°F), and Kelvin (K).
-
Celsius (°C): A widely used scale, particularly in scientific contexts, where 0°C represents the freezing point of water and 100°C represents the boiling point of water at standard atmospheric pressure.
-
Fahrenheit (°F): Primarily used in the United States, this scale sets the freezing point of water at 32°F and the boiling point at 212°F.
-
Kelvin (K): This is the absolute temperature scale, crucial in scientific work and thermodynamics. It's based on absolute zero, the theoretical temperature at which all molecular motion ceases. 0 K is absolute zero, and the size of a Kelvin degree is the same as a Celsius degree. This means a temperature change of 1°C is equivalent to a temperature change of 1 K. Therefore, to convert Celsius to Kelvin, you simply add 273.15.
Boiling Point: The Transition from Liquid to Gas
The boiling point is the temperature at which a liquid's vapor pressure equals the surrounding atmospheric pressure. At this point, bubbles of vapor form within the liquid, rise to the surface, and escape as gas. It's important to note that the boiling point is dependent on pressure. At higher altitudes, where atmospheric pressure is lower, liquids boil at lower temperatures. Conversely, at higher pressures, the boiling point increases.
Determining the Boiling Point in Kelvin: A Step-by-Step Guide
To find the boiling point of a substance in Kelvin, you need the boiling point in either Celsius or Fahrenheit. Here's how to convert:
1. If the boiling point is in Celsius (°C):
Add 273.15 to the Celsius value.
Formula: Kelvin (K) = Celsius (°C) + 273.15
Example: The boiling point of water at standard atmospheric pressure is 100°C. To convert to Kelvin:
K = 100°C + 273.15 = 373.15 K
Therefore, the boiling point of water is 373.15 K.
2. If the boiling point is in Fahrenheit (°F):
First, convert Fahrenheit to Celsius using the following formula:
Formula: Celsius (°C) = (°F - 32) × 5/9
Then, add 273.15 to the Celsius value to obtain the Kelvin equivalent.
Example: Let's say the boiling point of a substance is 212°F. First, convert to Celsius:
°C = (212°F - 32) × 5/9 = 100°C
Now, convert to Kelvin:
K = 100°C + 273.15 = 373.15 K
The Importance of Kelvin in Boiling Point Calculations
The Kelvin scale's significance in determining boiling points lies in its absolute nature. Using Kelvin avoids negative values, which can arise in Celsius or Fahrenheit, simplifying calculations and providing a more fundamental understanding of thermal processes. Many scientific formulas and equations related to thermodynamics and heat transfer require temperature to be expressed in Kelvin.
Boiling Points of Common Substances in Kelvin
Here's a table showing the boiling points of several common substances in Kelvin at standard atmospheric pressure:
| Substance | Boiling Point (°C) | Boiling Point (K) |
|---|---|---|
| Water | 100 | 373.15 |
| Ethanol | 78.4 | 351.55 |
| Oxygen | -183 | 90.15 |
| Nitrogen | -196 | 77.15 |
| Helium | -268.93 | 4.22 |
| Mercury | 356.7 | 629.85 |
Factors Affecting Boiling Point
Several factors can influence the boiling point of a substance:
1. Intermolecular Forces:
Stronger intermolecular forces (like hydrogen bonding in water) require more energy to overcome, resulting in higher boiling points.
2. Molecular Weight:
Heavier molecules generally have higher boiling points because they have stronger London dispersion forces.
3. Pressure:
As mentioned earlier, increased pressure leads to a higher boiling point, while decreased pressure leads to a lower boiling point. This is why water boils at a lower temperature at high altitudes.
4. Impurities:
The presence of impurities in a liquid can elevate its boiling point. This phenomenon is known as boiling point elevation.
Applications of Boiling Point Knowledge
Understanding boiling points is crucial in numerous fields:
-
Chemistry: Determining the purity of substances, conducting distillation processes, and understanding reaction mechanisms.
-
Physics: Studying phase transitions, thermodynamics, and heat transfer.
-
Engineering: Designing and operating various industrial processes, including distillation columns, power plants, and refrigeration systems.
-
Cooking: Understanding how different liquids behave at different temperatures during cooking.
-
Meteorology: Predicting weather patterns and understanding atmospheric processes.
Advanced Concepts: Critical Point and Supercritical Fluids
At extremely high temperatures and pressures, the distinction between liquid and gas phases disappears. This point is called the critical point. Beyond the critical point, the substance exists as a supercritical fluid, possessing properties of both liquids and gases. Understanding the critical point and supercritical fluids is essential in various advanced applications like supercritical fluid extraction.
Conclusion: The Significance of Kelvin in Boiling Point Determination
The boiling point is a fundamental physical property, and its accurate determination is critical in various scientific and engineering applications. Expressing the boiling point in Kelvin, the absolute temperature scale, provides a precise and universally understood measure, avoiding the ambiguities associated with relative scales like Celsius and Fahrenheit. Understanding the factors influencing boiling points and their practical applications is essential for anyone working in related fields. The concepts discussed in this article provide a solid foundation for further exploration into the fascinating world of phase transitions and thermodynamics.
Latest Posts
Latest Posts
-
Write The Chemical Formula For Chloric Acid
Mar 17, 2025
-
5 Letter Words With O W
Mar 17, 2025
-
The Number Of Elements In A Set Is Called The
Mar 17, 2025
-
What Is The Prime Factorization Of 59
Mar 17, 2025
-
What Is The Difference Between A Parallelogram And A Trapezoid
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point For Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
