What Is The Equivalence Point In A Titration
Juapaving
Mar 28, 2025 · 6 min read
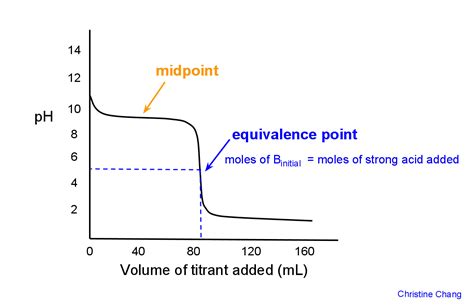
Table of Contents
What is the Equivalence Point in a Titration? A Comprehensive Guide
Titration, a cornerstone technique in analytical chemistry, allows for the precise determination of an unknown concentration of a substance. This process hinges on the concept of the equivalence point, a crucial juncture that signals the complete neutralization or reaction between the titrant (the solution of known concentration) and the analyte (the solution of unknown concentration). Understanding the equivalence point is paramount to accurate titration results. This comprehensive guide will delve into the intricacies of the equivalence point, exploring its definition, identification, factors influencing it, and its practical applications.
Defining the Equivalence Point
The equivalence point in a titration is the point at which the moles of titrant added are stoichiometrically equivalent to the moles of analyte present. This means the chemical reaction between the titrant and the analyte is complete. It's a theoretical point, representing the ideal completion of the reaction. It's important to distinguish this from the end point, which is the point at which the indicator changes color, signaling the approximate completion of the reaction. Ideally, the end point should be as close as possible to the equivalence point, but slight discrepancies can occur.
Think of it like baking a cake. The recipe specifies a precise ratio of ingredients – flour, sugar, eggs, etc. The equivalence point is analogous to achieving the perfect ratio where all ingredients have reacted completely, resulting in the ideal cake. The end point, in this analogy, is when you visually assess the cake’s readiness, which may differ slightly from the perfect ratio due to subjective observation.
Stoichiometry and the Equivalence Point
The stoichiometric relationship between the titrant and analyte is key to understanding the equivalence point. This relationship is defined by the balanced chemical equation for the reaction. For example, in a strong acid-strong base titration:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The stoichiometry is 1:1; one mole of HCl reacts completely with one mole of NaOH. At the equivalence point, the moles of HCl will equal the moles of NaOH.
However, the stoichiometry isn't always 1:1. Consider the titration of sulfuric acid (H₂SO₄) with sodium hydroxide (NaOH):
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
Here, the stoichiometry is 1:2; one mole of H₂SO₄ reacts with two moles of NaOH. The equivalence point is reached when twice the moles of NaOH have been added compared to the moles of H₂SO₄. This highlights the importance of considering the balanced chemical equation when calculating the equivalence point.
Identifying the Equivalence Point
Identifying the equivalence point precisely is crucial for accurate results. This isn't always straightforward and depends on various factors, including the type of titration and the presence of an indicator.
Methods for Equivalence Point Determination
Several techniques exist to determine the equivalence point, each with its advantages and limitations:
-
Visual Indicators: These are substances that change color near the equivalence point. The choice of indicator depends on the pH range of the equivalence point. For strong acid-strong base titrations, phenolphthalein is a common choice. However, visual indicators are subjective and can introduce some error.
-
pH Meter: A pH meter provides a continuous measurement of pH throughout the titration. Plotting the pH against the volume of titrant added creates a titration curve. The equivalence point is identified as the point of steepest slope (the inflection point) on the curve. This method offers higher accuracy than visual indicators.
-
Conductivity Measurement: The conductivity of the solution changes during the titration. The equivalence point can be identified as the point of minimum or maximum conductivity, depending on the nature of the titrant and analyte. This technique is particularly useful for titrations involving weak electrolytes.
-
Potentiometric Titration: This method uses an electrode sensitive to the concentration of one of the reactants or products in the titration. The potential difference between the electrode and a reference electrode is monitored, and the equivalence point is determined from the change in potential. This is a highly accurate method.
Factors Influencing the Equivalence Point
Several factors can influence the equivalence point's precise location and accurate determination:
-
Temperature: Temperature changes affect the equilibrium constant of the reaction, potentially shifting the equivalence point. Maintaining a constant temperature throughout the titration is crucial for accuracy.
-
Ionic Strength: High ionic strength can affect the activity of ions involved in the reaction, leading to deviations from the expected equivalence point.
-
Presence of Impurities: Impurities in the titrant or analyte can interfere with the reaction, altering the stoichiometry and shifting the equivalence point.
-
Indicator Choice: The indicator's pKa (acid dissociation constant) determines its color change range. If the indicator's color change range doesn't overlap sufficiently with the pH change around the equivalence point, an inaccurate determination can result. Proper indicator selection is critical for accurate results.
Titration Curves and Equivalence Point Determination
A titration curve is a graph that plots the change in a specific property (e.g., pH, conductivity) of the analyte solution against the volume of titrant added. Analyzing this curve is critical for identifying the equivalence point.
Strong Acid-Strong Base Titration Curves
These curves exhibit a sharp, vertical change in pH near the equivalence point, making it relatively easy to determine. The equivalence point occurs at pH 7.
Weak Acid-Strong Base Titration Curves
These curves show a gradual pH change near the equivalence point, making the identification less precise. The equivalence point occurs at a pH greater than 7.
Weak Base-Strong Acid Titration Curves
Similar to weak acid-strong base titrations, these curves exhibit a gradual pH change around the equivalence point, which is less than 7.
Polyprotic Acid Titration Curves
Polyprotic acids have multiple equivalence points, one for each proton donated. The titration curve will show multiple inflection points, each corresponding to an equivalence point.
Applications of Equivalence Point Determination
The concept of the equivalence point is central to numerous applications in chemistry and related fields:
-
Quantitative Analysis: Titration is extensively used to determine the concentration of unknown solutions, such as acids, bases, and oxidizing/reducing agents. This is crucial in various industries, including pharmaceuticals, environmental monitoring, and food science.
-
Acid-Base Chemistry: Determining the equivalence point is fundamental to understanding acid-base reactions and equilibrium. This is crucial in chemistry education and research.
-
Redox Titrations: Equivalence point determination is essential for redox titrations, where the titrant and analyte undergo oxidation-reduction reactions. These titrations find applications in determining the concentration of oxidizing and reducing agents.
-
Complexometric Titrations: Complexometric titrations involve the formation of stable complexes between the titrant and analyte. The equivalence point is determined by the change in the concentration of free metal ions.
-
Precipitation Titrations: Precipitation titrations involve the formation of a precipitate as the reaction proceeds. The equivalence point is determined by the change in the concentration of ions involved in the precipitation reaction.
Conclusion
The equivalence point is a cornerstone concept in titration, representing the complete reaction between the titrant and analyte. Understanding its definition, methods for its determination, and the factors influencing it is vital for accurate and reliable results. The equivalence point's determination allows for precise quantitative analysis, crucial across various scientific disciplines and industrial applications. From simple acid-base titrations to more complex redox and precipitation titrations, mastering the concept of the equivalence point is paramount for analytical chemists and anyone working with chemical solutions. Precise identification of the equivalence point, whether through visual indicators, pH meters, or other methods, underpins the success of many analytical procedures and allows for accurate and reliable results in a wide variety of settings.
Latest Posts
Latest Posts
-
Which Is Bigger Gb Or Mg
Mar 30, 2025
-
Why Is Cellulose Not A Source Of Nutrients For Humans
Mar 30, 2025
-
Law Of Conservation Of Mass States That
Mar 30, 2025
-
Simplify The Square Root Of 12
Mar 30, 2025
-
Find The Area Under The Curve Over The Interval
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Equivalence Point In A Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
