What Is The Empirical Formula Of C6h6
Juapaving
Mar 24, 2025 · 5 min read
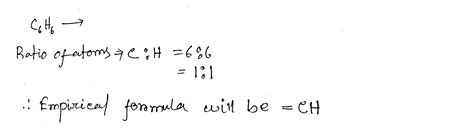
Table of Contents
What is the Empirical Formula of C₆H₆? Understanding Molecular and Empirical Formulas
The question, "What is the empirical formula of C₆H₆?" seems deceptively simple. The answer, at first glance, appears to be C₆H₆ itself. However, delving deeper reveals a crucial understanding of the difference between molecular and empirical formulas, and the importance of simplification in chemical representation. This article will explore this distinction, explaining how to determine the empirical formula, its significance, and the unique case of benzene (C₆H₆).
Understanding Molecular and Empirical Formulas
Before tackling the specific case of C₆H₆, let's establish a clear understanding of molecular and empirical formulas. These two representations convey different aspects of a molecule's composition.
Molecular Formula: This formula precisely indicates the actual number of atoms of each element present in a single molecule. For example, the molecular formula of glucose is C₆H₁₂O₆, meaning each glucose molecule contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Empirical Formula: This formula represents the simplest whole-number ratio of atoms of each element in a compound. It's the most reduced form of the molecular formula. It doesn't necessarily reflect the actual number of atoms in a molecule, but rather the ratio between them. To illustrate, the empirical formula for glucose (C₆H₁₂O₆) is CH₂O, because the ratio of carbon to hydrogen to oxygen is 1:2:1.
Determining the Empirical Formula: A Step-by-Step Guide
The process of determining the empirical formula typically involves the following steps:
-
Determine the mass of each element present: This is usually done through experimental methods like combustion analysis or elemental analysis. The results provide the mass of each element in a sample of the compound.
-
Convert the mass of each element to moles: Use the molar mass (atomic weight) of each element to convert the mass (in grams) to moles. The molar mass is found on the periodic table.
-
Divide each mole value by the smallest mole value: This step normalizes the mole ratios, providing the simplest whole-number ratio of atoms.
-
Multiply the ratios by an integer (if necessary): If the ratios obtained in step 3 are not whole numbers, multiply each ratio by the smallest integer that will convert all ratios to whole numbers.
-
Write the empirical formula: Use the whole-number ratios obtained in step 4 to write the empirical formula, with the elements listed in order.
The Case of Benzene (C₆H₆): A Unique Scenario
Now, let's return to the original question: What is the empirical formula of C₆H₆ (benzene)?
The molecular formula of benzene is indeed C₆H₆. To find the empirical formula, we follow the steps outlined above:
-
Mass of each element: We already have the number of atoms, implicitly indicating the mass ratio.
-
Convert to moles: This step is unnecessary in this case because we already have the number of atoms.
-
Divide by the smallest mole value: The smallest number of atoms is 6 (for both carbon and hydrogen). Dividing both 6 and 6 by 6 yields 1 and 1.
-
Multiply ratios by an integer: The ratios are already whole numbers (1:1).
-
Write the empirical formula: The empirical formula is CH.
Therefore, even though the molecular formula of benzene is C₆H₆, its empirical formula is CH. This highlights the fundamental difference between these two representations. The empirical formula tells us the simplest ratio of carbon to hydrogen atoms, while the molecular formula indicates the actual number of atoms in a benzene molecule.
Significance of Empirical and Molecular Formulas
Both empirical and molecular formulas play crucial roles in chemistry:
-
Empirical Formula: Useful for determining the simplest composition of a compound, particularly useful when dealing with unknown compounds where the exact molecular formula is yet to be determined. It provides the foundational information about the elemental composition. It is essential in determining the percentage composition of elements in a compound.
-
Molecular Formula: Provides the precise composition of a molecule, critical for understanding its structure, properties, and chemical reactions. It is the basis for stoichiometric calculations in chemical reactions, including balancing equations.
Beyond Benzene: Examples of Molecular and Empirical Formulas
Let's examine a few more examples to reinforce the understanding of molecular and empirical formulas:
-
Water (H₂O): The molecular formula is H₂O, and the empirical formula is also H₂O. The ratio of hydrogen to oxygen is already in its simplest form.
-
Ethane (C₂H₆): The molecular formula is C₂H₆, while the empirical formula is CH₃. The ratio of carbon to hydrogen is simplified to 1:3.
-
Hydrogen Peroxide (H₂O₂): The molecular formula is H₂O₂, and the empirical formula is HO. The simplest whole-number ratio of hydrogen to oxygen is 1:1.
-
Glucose (C₆H₁₂O₆): As mentioned earlier, the molecular formula is C₆H₁₂O₆, and the empirical formula is CH₂O.
Conclusion: Empirical Formula as a Building Block
In conclusion, while the molecular formula of benzene is C₆H₆, its empirical formula is CH. This seemingly simple example underscores the importance of understanding the distinction between molecular and empirical formulas. The empirical formula represents the simplest ratio of atoms, providing a fundamental understanding of the compound's composition. It forms the basis for further analysis and determination of the molecular formula, which provides the complete and accurate description of the molecule’s structure and properties. Understanding both is essential for navigating the world of chemistry effectively. The case of benzene serves as a valuable illustration of how these concepts work together to offer a comprehensive picture of a molecule's structure and composition. This distinction is a crucial foundational concept in chemistry, applicable to countless compounds and reactions.
Latest Posts
Latest Posts
-
Adjectives That Start With A D
Mar 26, 2025
-
Newtons Third Law Real Life Examples
Mar 26, 2025
-
What Is The Part Of Speech Of For
Mar 26, 2025
-
Whats The Square Root Of 125
Mar 26, 2025
-
What Is The Smallest Multiple Of 3 And 4
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula Of C6h6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
