What Is The Empirical Formula For Benzene C6h6
Juapaving
Mar 22, 2025 · 5 min read
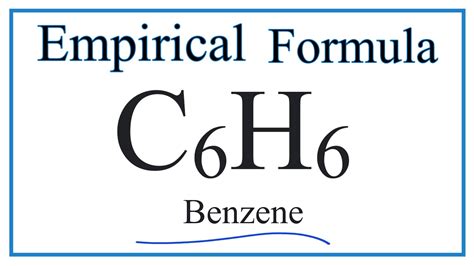
Table of Contents
What is the Empirical Formula for Benzene (C₆H₆)? Understanding Molecular and Empirical Formulas
Benzene, a ubiquitous aromatic hydrocarbon with the chemical formula C₆H₆, is a fascinating molecule that has captivated chemists for centuries. Its unique structure and properties have led to significant advancements in organic chemistry and countless industrial applications. Understanding its chemical formula, specifically the distinction between its molecular and empirical formulas, is crucial to grasping its chemical behavior. This article will delve into the intricacies of benzene's formula, exploring the concept of empirical formulas, explaining why the empirical formula isn't sufficient for describing benzene, and highlighting the importance of its molecular formula in understanding its reactivity and properties.
Understanding Empirical Formulas
The empirical formula of a chemical compound represents the simplest whole-number ratio of atoms of each element present in the compound. It shows the relative proportions of the different elements, but it doesn't necessarily reflect the actual number of atoms in a molecule. The empirical formula is determined experimentally through techniques like elemental analysis, which measures the mass percentages of each element in a sample.
To illustrate, let's consider a simpler example. If elemental analysis reveals that a compound contains 75% carbon and 25% hydrogen by mass, we can determine its empirical formula. Assuming a 100g sample, we have 75g of carbon and 25g of hydrogen. Converting these masses to moles using the atomic masses (C = 12 g/mol, H = 1 g/mol), we get approximately 6.25 moles of carbon and 25 moles of hydrogen. Dividing both by the smallest number of moles (6.25), we obtain a carbon-to-hydrogen ratio of roughly 1:4. Therefore, the empirical formula is CH₄ (methane).
Benzene's Molecular Formula: C₆H₆
While the empirical formula provides a simplified representation of the atom ratios, it often fails to capture the actual molecular structure. Benzene, with its molecular formula C₆H₆, perfectly exemplifies this limitation. The empirical formula for benzene, derived from the simplest whole-number ratio of carbon and hydrogen atoms, is also CH. This is because the ratio of carbon to hydrogen atoms in benzene is 1:1. However, this drastically underrepresents the molecule's true complexity and its properties.
Why the Empirical Formula is Insufficient for Benzene
The empirical formula CH for benzene is insufficient because it doesn't reflect the molecule's actual structure or its unique properties. Benzene's unique aromatic character, its stability, and its reactivity are all intimately linked to its molecular structure, which is significantly more complex than what the empirical formula suggests. The empirical formula fails to convey the following critical aspects:
- Cyclic Structure: The empirical formula CH gives no indication that benzene is a cyclic compound, meaning its atoms are arranged in a ring.
- Unsaturation: Benzene contains a significant degree of unsaturation, indicated by the presence of multiple bonds within the ring structure. The empirical formula doesn't reflect this.
- Resonance Stabilization: The special stability of benzene arises from resonance, a phenomenon where electrons are delocalized across the ring, creating a highly stable structure. This is entirely absent from the information provided by the empirical formula.
- Aromaticity: Benzene is an aromatic compound, a class of cyclic compounds with unique properties due to their delocalized pi electron system. This critical property is completely obscured by the simplified empirical formula.
Therefore, solely using the empirical formula for benzene would lead to a profound misunderstanding of its true chemical nature and behavior.
Determining the Molecular Formula of Benzene: Beyond Elemental Analysis
Determining benzene's molecular formula requires more than just elemental analysis. Additional techniques, such as mass spectrometry and other spectroscopic methods, are needed to establish the actual number of atoms in a molecule. Mass spectrometry, for instance, directly measures the molecular mass of the compound, providing crucial information for determining the molecular formula.
The Significance of Benzene's Molecular Formula (C₆H₆)
The molecular formula C₆H₆ is essential for understanding the unique properties and reactivity of benzene. It allows chemists to:
- Predict Chemical Behavior: Knowing the molecular formula enables prediction of benzene's reactions based on its bonding characteristics and electron distribution. For example, it explains why benzene undergoes electrophilic aromatic substitution rather than addition reactions, a hallmark of aromatic systems.
- Understand its Structure: The molecular formula, coupled with structural determination techniques, clarifies the cyclic structure, the presence of alternating single and double bonds (although it's more accurate to describe it as a resonance hybrid with delocalized electrons), and the planarity of the molecule.
- Explain its Stability: The molecular formula helps explain benzene's remarkable stability, which is much greater than expected for a hypothetical molecule with three isolated double bonds. This enhanced stability is due to the resonance stabilization resulting from the delocalized pi electron system.
- Develop Applications: The understanding derived from the molecular formula fuels the development of countless applications of benzene and its derivatives, from plastics and polymers to pharmaceuticals and dyes.
Benzene's Resonance Structures and its Implications
Benzene's structure is often represented by two resonance structures, each showing alternating single and double bonds in the ring. However, it's crucial to understand that neither structure accurately depicts the molecule; instead, they represent a resonance hybrid, a blend of these contributing structures where the electrons are delocalized across the entire ring. This delocalization accounts for benzene's exceptional stability and explains its behavior in chemical reactions.
Conclusion: Empirical vs. Molecular Formula – A Crucial Distinction
While the empirical formula provides a simplified ratio of elements, it's inadequate for fully describing the complexity of a molecule like benzene. For benzene, the molecular formula C₆H₆ is crucial. It reveals the true structure and, consequently, the unique aromatic character, reactivity, and stability that dictate its properties and numerous applications. Understanding the difference between empirical and molecular formulas is a fundamental step in comprehending the intricacies of organic chemistry and the behavior of chemical compounds. Benzene serves as a prime example of how a simplified empirical formula can mask the profound chemical reality conveyed by the molecular formula. The wealth of information contained within the molecular formula C₆H₆ underpins the significant role of benzene in both theoretical and applied chemistry.
Latest Posts
Latest Posts
-
What Is Larger 3 8 Or 1 2
Mar 23, 2025
-
What Domain Is Kingdom Animalia In
Mar 23, 2025
-
Functional And Structural Unit Of Kidney
Mar 23, 2025
-
Which Number Is Divisible By 6
Mar 23, 2025
-
What Are Parts Of A Fraction
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula For Benzene C6h6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
