What Is The Electron Configuration For Ni2+
Juapaving
Mar 24, 2025 · 6 min read
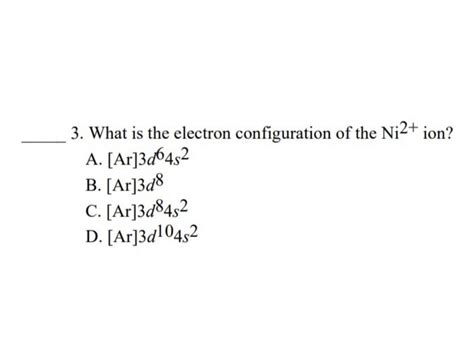
Table of Contents
What is the Electron Configuration for Ni²⁺? A Deep Dive into Nickel(II) Ion
Understanding electron configuration is fundamental to comprehending the properties and behavior of elements and their ions. This article delves deep into the electron configuration of the nickel(II) ion (Ni²⁺), explaining the process of determining it, its implications for chemical reactivity, and its relevance in various applications.
Understanding Electron Configuration
Before we explore the specifics of Ni²⁺, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. These arrangements are governed by the principles of quantum mechanics, which dictate that electrons occupy orbitals with specific energy levels and shapes. The filling of these orbitals follows the Aufbau principle (building-up principle), Hund's rule, and the Pauli exclusion principle.
- Aufbau Principle: Electrons fill orbitals from the lowest energy level to the highest.
- Hund's Rule: Within a subshell, electrons will individually occupy each orbital before pairing up.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers (n, l, ml, ms). This means each orbital can hold a maximum of two electrons with opposite spins.
The electron configuration is typically represented using a notation that indicates the principal quantum number (n), the subshell (s, p, d, f), and the number of electrons in each subshell. For example, the electron configuration of a neutral nickel atom (Ni) is [Ar] 3d⁸ 4s².
Determining the Electron Configuration of Ni²⁺
Nickel (Ni) is a transition metal with an atomic number of 28. This means a neutral nickel atom has 28 electrons. The Ni²⁺ ion is formed when a neutral nickel atom loses two electrons. The crucial question is: which two electrons are lost?
The general rule for transition metals is that electrons are first removed from the s subshell before the d subshell. This is because the 4s subshell, although higher in principal quantum number, has a slightly lower energy level than the 3d subshell in many transition metals. Therefore, the two electrons lost from nickel to form the Ni²⁺ ion are the two electrons in the 4s orbital.
Therefore, the electron configuration of Ni²⁺ is [Ar] 3d⁸.
Step-by-Step Breakdown:
-
Neutral Nickel (Ni): The electron configuration of a neutral nickel atom is [Ar] 3d⁸ 4s².
-
Ionization: To form Ni²⁺, two electrons are removed.
-
Electron Removal: The two electrons from the 4s orbital are removed first.
-
Final Configuration: The resulting electron configuration of Ni²⁺ is [Ar] 3d⁸. This means the ion now has 26 electrons.
Implications of the Ni²⁺ Electron Configuration
The 3d⁸ electron configuration of Ni²⁺ has significant implications for its chemical and physical properties.
Magnetic Properties:
The presence of unpaired electrons in the 3d orbitals makes Ni²⁺ paramagnetic. Paramagnetic substances are weakly attracted to magnetic fields due to the presence of unpaired electrons. The eight electrons in the 3d orbitals of Ni²⁺ occupy four orbitals individually before pairing, leaving two unpaired electrons. This paramagnetism is a key characteristic that can be used to identify Ni²⁺ in various analytical techniques.
Coordination Chemistry:
Ni²⁺ is a highly versatile ion in coordination chemistry, forming a wide range of complexes with various ligands. The d⁸ configuration allows Ni²⁺ to exhibit different geometries (tetrahedral, square planar, octahedral) depending on the ligand field strength. The ligand field theory explains how the interaction between the metal ion and the ligands affects the energies of the d orbitals, leading to different complex geometries and properties. The specific arrangement of ligands around the Ni²⁺ ion influences its color, reactivity, and magnetic properties. Examples of Ni²⁺ complexes include [Ni(H₂O)₆]²⁺ (octahedral), and [NiCl₄]²⁻ (tetrahedral).
Catalytic Activity:
Nickel and its compounds, including Ni²⁺, exhibit significant catalytic activity in various chemical reactions. The ability of Ni²⁺ to readily change its oxidation state and participate in redox reactions makes it a valuable catalyst in numerous industrial processes, such as hydroformylation, hydrogenation, and cross-coupling reactions. Its partially filled d orbitals facilitate the interaction with reactant molecules, lowering the activation energy of the reactions.
Biological Roles:
Although not as prevalent as some other transition metals, nickel plays a role in certain biological systems. Certain enzymes, known as nickel enzymes, require nickel ions for their catalytic activity. These enzymes are involved in various metabolic processes, such as methane metabolism and carbon monoxide oxidation. The specific role of Ni²⁺ within these enzymes often relies on its ability to form coordination complexes with specific amino acid residues within the protein structure.
Spectroscopic Properties of Ni²⁺ Compounds
The electronic configuration and coordination environment of Ni²⁺ significantly influence its spectroscopic properties. The d-d transitions, which are electronic transitions within the d orbitals, are responsible for the characteristic colors of Ni²⁺ complexes. The energy difference between the d orbitals, determined by the ligand field, dictates the wavelength of light absorbed, resulting in the complementary color being observed. For example, [Ni(H₂O)₆]²⁺ appears green due to the absorption of red light.
Techniques such as UV-Vis spectroscopy and electron paramagnetic resonance (EPR) spectroscopy are commonly used to study the electronic structure and coordination environment of Ni²⁺ complexes. These spectroscopic methods provide valuable information about the energy levels, symmetry, and magnetic properties of these compounds.
Applications of Ni²⁺ Compounds
The unique properties of Ni²⁺ have led to a wide range of applications across various fields:
-
Catalysis: As mentioned earlier, Ni²⁺ is a crucial component in many industrial catalysts used in various chemical processes.
-
Batteries: Nickel-based batteries, such as nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries, are widely used in portable electronic devices and electric vehicles. The redox reactions involving Ni²⁺ are vital for the energy storage and release processes in these batteries.
-
Coatings: Nickel plating is frequently used to enhance the corrosion resistance and durability of metallic components. The protective Ni²⁺ layer shields the underlying metal from oxidation and other forms of deterioration.
-
Alloys: Nickel alloys exhibit excellent mechanical properties, including high strength and corrosion resistance, and are widely used in various industrial applications, including aerospace components and high-temperature applications.
Conclusion: The Significance of Understanding Ni²⁺
The electron configuration of Ni²⁺, [Ar] 3d⁸, is not simply a theoretical concept; it is the foundation for understanding the diverse and important properties of this ion. From its magnetic behavior and coordination chemistry to its catalytic activity and various applications, the 3d⁸ configuration is intrinsically linked to the practical significance of nickel(II) in science, technology, and industry. Further research continues to explore the unique interactions of Ni²⁺, unlocking new potential for innovative applications and deeper fundamental understanding. Understanding the electron configuration of ions like Ni²⁺ is crucial for developing new materials, catalysts, and technologies that improve our lives. The continued study of transition metal ions and their electron configurations will undoubtedly drive future advancements across many scientific and technological fields.
Latest Posts
Latest Posts
-
Line Of Best Fit Equation Calculator
Mar 26, 2025
-
What Is The Half Of 24
Mar 26, 2025
-
Is The Number 23 Prime Or Composite
Mar 26, 2025
-
Is Square Root Of 15 A Rational Number
Mar 26, 2025
-
Which Of These Is A Polysaccharide
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Ni2+ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
