What Is The Difference Between Molecular Geometry And Electron Geometry
Juapaving
Mar 30, 2025 · 6 min read
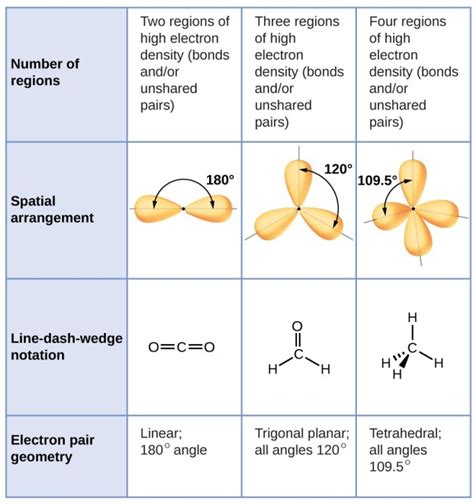
Table of Contents
What's the Difference Between Molecular Geometry and Electron Geometry?
Understanding the three-dimensional arrangement of atoms within a molecule is crucial in chemistry. This understanding helps predict a molecule's properties, reactivity, and overall behavior. Two closely related yet distinct concepts often cause confusion: molecular geometry and electron geometry. While both describe the spatial arrangement of atoms and electron pairs, they focus on different aspects. This article delves deep into the differences between molecular geometry and electron geometry, providing clear explanations and examples to solidify your understanding.
Understanding Electron Geometry: The Big Picture
Electron geometry describes the arrangement of all electron pairs surrounding the central atom in a molecule, including both bonding pairs (pairs involved in covalent bonds) and lone pairs (non-bonding pairs). It considers all electron domains, regardless of whether they are involved in bonding. We utilize the Valence Shell Electron Pair Repulsion (VSEPR) theory to predict electron geometry. VSEPR theory posits that electron pairs repel each other and will arrange themselves to minimize this repulsion, resulting in specific geometric shapes.
Key Factors Influencing Electron Geometry:
- Number of electron domains: This is the sum of bonding pairs and lone pairs around the central atom.
- Repulsion forces: Lone pairs exert a stronger repulsive force than bonding pairs, influencing the overall geometry.
Common Electron Geometries:
- Linear: Two electron domains (e.g., BeCl₂). A straight line is formed by the electron pairs.
- Trigonal Planar: Three electron domains (e.g., BF₃). The electron pairs are arranged in a flat triangle.
- Tetrahedral: Four electron domains (e.g., CH₄). The electron pairs are positioned at the corners of a tetrahedron.
- Trigonal Bipyramidal: Five electron domains (e.g., PCl₅). A combination of a triangular base and two axial positions.
- Octahedral: Six electron domains (e.g., SF₆). The electron pairs occupy the corners of an octahedron.
Molecular Geometry: Focusing on the Atoms
Molecular geometry, also known as molecular shape, describes the arrangement of only the atoms in a molecule. It differs from electron geometry because it only considers the positions of the atoms, ignoring the lone pairs on the central atom. Lone pairs influence the overall shape but are not included in the description of the molecular geometry itself.
The Role of Lone Pairs in Molecular Geometry:
While lone pairs are not part of the description of molecular geometry, they significantly affect the actual shape. They exert a stronger repulsive force than bonding pairs, causing the bonding pairs and hence the atoms to be slightly pushed closer together. This leads to deviations from the ideal electron geometry.
Common Molecular Geometries:
Many molecular geometries correspond directly to their electron geometries when there are no lone pairs. However, the presence of lone pairs leads to distortions.
- Linear: Same as electron geometry (e.g., BeCl₂). Two atoms bonded to a central atom, forming a straight line.
- Trigonal Planar: Same as electron geometry if all bonds are present (e.g., BF₃). Three atoms bonded to a central atom, forming a flat triangle.
- Bent (V-shaped): Derived from a tetrahedral electron geometry with two lone pairs (e.g., H₂O). The two bonding pairs are pushed closer together, creating a bent shape.
- Trigonal Pyramidal: Derived from a tetrahedral electron geometry with one lone pair (e.g., NH₃). Three atoms are bonded to a central atom forming a triangular pyramid.
- Tetrahedral: Same as electron geometry if no lone pairs (e.g., CH₄). Four atoms bonded to a central atom forming a tetrahedron.
- See-saw: Derived from a trigonal bipyramidal electron geometry (e.g., SF₄). The lone pairs influence the shape, creating a see-saw structure.
- T-shaped: Derived from a trigonal bipyramidal electron geometry (e.g., ClF₃). Two lone pairs affect the arrangement of the three atoms bonded to the central atom, resulting in a T-shape.
- Linear (from trigonal bipyramidal): Derived from a trigonal bipyramidal electron geometry (e.g., I₃⁻). Two lone pairs affect the shape, resulting in a linear structure.
- Square Planar: Derived from an octahedral electron geometry (e.g., XeF₄). Two lone pairs influence the arrangement of the four bonded atoms, leading to a square planar shape.
- Square Pyramidal: Derived from an octahedral electron geometry (e.g., BrF₅). One lone pair affects the shape, resulting in a square pyramid.
Comparing Electron Geometry and Molecular Geometry: A Table Summary
| Feature | Electron Geometry | Molecular Geometry |
|---|---|---|
| Focus | Arrangement of all electron pairs (bonding & lone) | Arrangement of atoms only |
| VSEPR Theory | Directly applied | Influenced by, but not directly determined by |
| Lone Pairs | Included in the determination of geometry | Affect the shape but are not included in the description |
| Predictive Power | Predicts the overall spatial arrangement of electrons | Predicts the actual three-dimensional shape of the molecule |
| Examples | Linear, Trigonal Planar, Tetrahedral, Trigonal Bipyramidal, Octahedral | Linear, Bent, Trigonal Pyramidal, Tetrahedral, See-saw, T-shaped, Square Planar, Square Pyramidal |
Illustrative Examples: Water (H₂O) and Methane (CH₄)
Let's illustrate the difference with two simple examples: water (H₂O) and methane (CH₄).
Water (H₂O):
-
Electron Geometry: The central oxygen atom has four electron domains: two bonding pairs (O-H bonds) and two lone pairs. The electron geometry is tetrahedral.
-
Molecular Geometry: The molecular geometry considers only the positions of the oxygen and hydrogen atoms. The two lone pairs repel the bonding pairs, causing the H-O-H bond angle to be less than the ideal 109.5° of a tetrahedron, resulting in a bent or V-shaped molecular geometry.
Methane (CH₄):
-
Electron Geometry: The central carbon atom has four electron domains, all bonding pairs (C-H bonds). The electron geometry is tetrahedral.
-
Molecular Geometry: Since all four electron domains are bonding pairs, the molecular geometry is also tetrahedral. There are no lone pairs to distort the shape.
The Importance of Understanding Both Geometries
Knowing both electron geometry and molecular geometry is crucial for several reasons:
-
Predicting Properties: Molecular geometry directly impacts the properties of a molecule, such as polarity, boiling point, melting point, and reactivity. For instance, the bent shape of water makes it a polar molecule, while the tetrahedral shape of methane makes it nonpolar.
-
Understanding Reactivity: The spatial arrangement of atoms determines where other molecules can approach and interact. This is critical in understanding chemical reactions.
-
Spectroscopic Analysis: Molecular geometry influences spectroscopic techniques like infrared (IR) and Raman spectroscopy.
Conclusion: A Clear Distinction
While often used interchangeably, electron geometry and molecular geometry are distinct concepts. Electron geometry considers all electron domains around a central atom, while molecular geometry focuses solely on the arrangement of atoms. Understanding both is essential for a comprehensive understanding of molecular structure and its impact on chemical properties and reactivity. By applying VSEPR theory and considering the influence of lone pairs, one can accurately predict both electron and molecular geometries and subsequently, interpret a molecule's properties and behavior. Mastering these concepts is a cornerstone of success in chemistry.
Latest Posts
Latest Posts
-
Is Chlorine A Pure Substance Or Mixture
Apr 01, 2025
-
How Do You Write 19 In Roman Numerals
Apr 01, 2025
-
Why Is It Warmer When It Snows
Apr 01, 2025
-
A Positively Charged Ion Is Called
Apr 01, 2025
-
What Is This Phenomenon Known As
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Molecular Geometry And Electron Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
