A Positively Charged Ion Is Called
Juapaving
Apr 01, 2025 · 7 min read
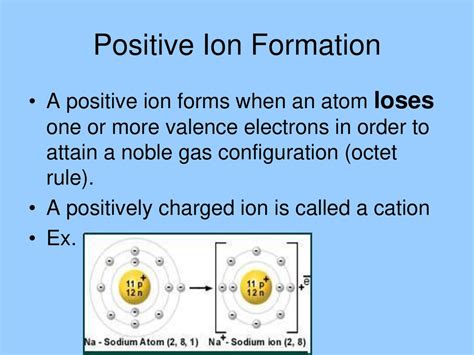
Table of Contents
A Positively Charged Ion is Called a Cation: A Deep Dive into Ionic Chemistry
A positively charged ion is called a cation. Understanding cations is fundamental to grasping many concepts in chemistry, physics, and biology. This comprehensive guide explores the formation, properties, and significance of cations, delving into their roles in various fields. We'll also explore related concepts like anions and ionic bonding to provide a complete picture of ionic chemistry.
What is a Cation?
A cation is an atom or molecule that has lost one or more electrons, resulting in a net positive charge. This positive charge arises because the number of protons (positively charged particles in the nucleus) now exceeds the number of electrons (negatively charged particles orbiting the nucleus). The process of forming a cation is called ionization, often occurring through chemical reactions or interactions with other charged particles.
The magnitude of the positive charge on a cation is denoted by a superscript plus sign (+). For example, a sodium ion (Na⁺) has a +1 charge, meaning it has lost one electron. A calcium ion (Ca²⁺) has a +2 charge, indicating the loss of two electrons. The charge on a cation is crucial in determining its chemical behavior and interactions with other atoms and molecules.
How are Cations Formed?
Cations are primarily formed through the loss of electrons from an atom's outermost electron shell, also known as the valence shell. This loss of electrons occurs to achieve a more stable electron configuration, often fulfilling the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons. Atoms with low ionization energies readily lose electrons and form cations. These are typically metals, located on the left-hand side of the periodic table.
For instance, sodium (Na) has one electron in its valence shell. By losing this single electron, it achieves a stable electron configuration similar to neon (Ne), a noble gas with a full outer shell. This results in the formation of a sodium cation (Na⁺).
Similarly, magnesium (Mg) has two electrons in its valence shell. To attain stability, it loses both electrons, forming a magnesium cation (Mg²⁺). The ease with which an atom loses electrons depends on factors such as its atomic number, electron configuration, and effective nuclear charge.
Key Properties of Cations
Cations possess several distinct properties that differentiate them from neutral atoms and anions (negatively charged ions):
-
Positive Charge: The defining characteristic of a cation is its net positive electrical charge. This charge arises from the imbalance between protons and electrons.
-
Smaller Size: When an atom loses electrons, it effectively reduces the electron cloud shielding the positive charge of the nucleus. This results in a decrease in the atomic radius, making cations smaller than their corresponding neutral atoms.
-
High Electronegativity: While not as high as anions, cations often exhibit relatively high electronegativity compared to their neutral counterparts. This means they have a strong tendency to attract electrons.
-
Reactivity: Cations are chemically reactive, particularly with anions. Their positive charge enables them to form strong ionic bonds with negatively charged species. The reactivity depends on the charge and size of the cation, as well as the properties of the anion it interacts with.
-
Metallic Character: Many cations are derived from metal atoms, inheriting some metallic properties. However, the extent of metallic character may vary depending on the specific cation and its environment.
Examples of Common Cations
Numerous cations exist, ranging from simple monoatomic ions to complex polyatomic ions. Here are a few common examples:
- Sodium ion (Na⁺): Found extensively in biological systems and used in table salt.
- Potassium ion (K⁺): Crucial for nerve impulse transmission and muscle contraction.
- Calcium ion (Ca²⁺): Essential for bone structure, muscle function, and blood clotting.
- Magnesium ion (Mg²⁺): Plays a vital role in enzymatic activity and chlorophyll structure in plants.
- Iron(II) ion (Fe²⁺) and Iron(III) ion (Fe³⁺): Involved in oxygen transport in hemoglobin and various metabolic processes.
- Ammonium ion (NH₄⁺): A polyatomic cation crucial in fertilizers and biological systems.
Cations in Ionic Bonding
Cations play a crucial role in ionic bonding, a type of chemical bond formed through the electrostatic attraction between oppositely charged ions. When a metal atom (which tends to lose electrons and form cations) interacts with a non-metal atom (which tends to gain electrons and form anions), the transfer of electrons leads to the formation of ions. The electrostatic force of attraction between the cation and the anion holds them together, forming an ionic compound.
For example, in sodium chloride (NaCl), commonly known as table salt, sodium (Na) loses one electron to form a sodium cation (Na⁺), while chlorine (Cl) gains one electron to form a chloride anion (Cl⁻). The electrostatic attraction between the positively charged Na⁺ and the negatively charged Cl⁻ ions forms the ionic bond.
The strength of the ionic bond is directly proportional to the magnitudes of the charges on the cation and anion and inversely proportional to the distance between them. Therefore, cations with higher charges tend to form stronger ionic bonds.
Cations in Various Fields
Cations are not just theoretical entities; they have significant practical applications across diverse fields:
Biology and Medicine:
Cations are essential for life. Many biological processes depend on the presence and proper balance of various cations, including sodium, potassium, calcium, and magnesium. These ions are vital for nerve impulse transmission, muscle contraction, enzyme activity, and many other cellular processes. Imbalances in cation levels can lead to serious health problems. For example, potassium imbalances can cause heart arrhythmias, and calcium imbalances can result in muscle spasms or weakness.
Chemistry and Materials Science:
Cations are fundamental building blocks in various materials. They are critical components in ceramics, glasses, and other inorganic materials, influencing their properties such as strength, hardness, and electrical conductivity. The study of cation behavior in these materials is crucial for designing and improving new materials with specific properties.
Environmental Science:
Cations play important roles in environmental processes. The presence and concentration of certain cations in soil and water influence nutrient availability to plants and the overall ecosystem health. The presence of heavy metal cations like lead (Pb²⁺) and mercury (Hg²⁺) can cause significant environmental pollution and pose health risks.
Industrial Applications:
Cations are utilized in various industrial processes. They are employed in electroplating, where a thin layer of metal is deposited onto a surface, and in batteries, where they participate in electrochemical reactions to generate electricity.
Understanding Anions: The Counterpart to Cations
To fully grasp the concept of cations, it's essential to understand anions, which are negatively charged ions. Anions are formed when an atom gains one or more electrons, resulting in an excess of negative charge. They often arise from non-metal atoms which have a high electron affinity and tend to attract electrons to fill their outermost electron shell. Examples include chloride (Cl⁻), oxide (O²⁻), and sulfate (SO₄²⁻) ions. Anions and cations always occur together in ionic compounds to ensure electrical neutrality. The overall charge of a stable ionic compound is always zero.
Conclusion: The Importance of Cations
In conclusion, a positively charged ion is called a cation. Cations are fundamental to understanding ionic chemistry and have far-reaching implications in various scientific fields. Their properties, formation, and interactions with other ions shape the characteristics of many materials and biological processes. Understanding cations is crucial for advancements in medicine, materials science, environmental science, and numerous other areas. The study of cations continues to be a vibrant area of research, driving innovations and discoveries across a wide spectrum of disciplines. Their significance underscores the importance of studying ionic interactions and the fundamental principles of chemical bonding. Further exploration into specific cation behaviors and their applications within specialized fields can lead to a richer understanding of their multifaceted roles in the world around us.
Latest Posts
Latest Posts
-
Which Statements Are True Regarding Undefinable Terms In Geometry
Apr 02, 2025
-
What Is 3 Of 1 Million
Apr 02, 2025
-
In What Cell Organelle Does Photosynthesis Occur
Apr 02, 2025
-
Name 3 Kinds Of Hard Part Fossils
Apr 02, 2025
-
Convert 100 Degrees Celsius To Fahrenheit
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Positively Charged Ion Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
