What Is The Difference Between Molecular And Electron Geometry
Juapaving
Mar 28, 2025 · 6 min read
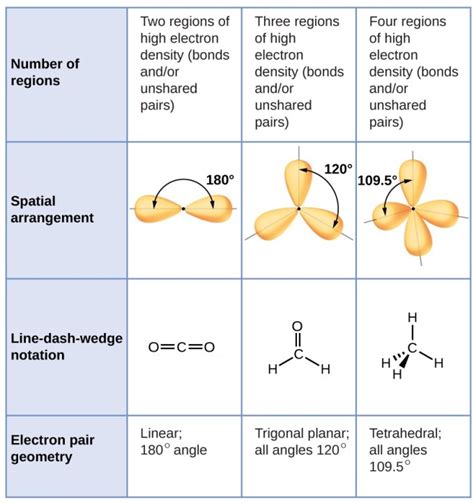
Table of Contents
What's the Difference Between Molecular and Electron Geometry?
Understanding the three-dimensional arrangement of atoms within a molecule is crucial in chemistry. This arrangement dictates many of the molecule's physical and chemical properties, from its reactivity to its boiling point. Two key concepts in describing this arrangement are molecular geometry and electron geometry. While closely related, they are distinct and understanding their differences is paramount to grasping the fundamentals of chemical bonding.
Understanding Electron Geometry: The Role of All Electron Pairs
Electron geometry describes the arrangement of all electron pairs surrounding the central atom in a molecule. This includes both bonding pairs (electrons shared between atoms in covalent bonds) and lone pairs (electrons not involved in bonding). The electron geometry is determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory, which posits that electron pairs repel each other and will arrange themselves to minimize this repulsion. This leads to predictable geometries based on the number of electron pairs around the central atom.
Key Geometries Based on Electron Pairs:
- Linear (2 electron pairs): Two electron pairs arranged 180° apart. Example: BeCl₂
- Trigonal Planar (3 electron pairs): Three electron pairs arranged 120° apart in a plane. Example: BF₃
- Tetrahedral (4 electron pairs): Four electron pairs arranged 109.5° apart in a three-dimensional tetrahedron. Example: CH₄
- Trigonal Bipyramidal (5 electron pairs): Five electron pairs arranged in a trigonal bipyramidal shape, with three pairs in a plane (120° apart) and two pairs perpendicular to this plane (90° and 180° from the planar pairs). Example: PCl₅
- Octahedral (6 electron pairs): Six electron pairs arranged 90° apart in an octahedron. Example: SF₆
It's crucial to remember that electron geometry considers all electron pairs, regardless of whether they are bonding or non-bonding. This is where it differs significantly from molecular geometry.
Molecular Geometry: Focusing on Atom Positions
Molecular geometry, on the other hand, describes the arrangement of only the atoms within a molecule. It focuses solely on the positions of the bonded atoms, ignoring the lone pairs. While the electron geometry dictates the overall arrangement of electron pairs, the molecular geometry is shaped by the spatial distribution of the atoms, which is influenced by both bonding and non-bonding electron pairs.
The Influence of Lone Pairs on Molecular Geometry:
Lone pairs, while not directly involved in the molecular geometry, exert a significant influence due to their repulsion. Because lone pairs are closer to the central atom than bonding pairs, they exert a stronger repulsive force. This causes a distortion in the bond angles and consequently alters the molecular geometry compared to the electron geometry.
Common Molecular Geometries:
The molecular geometries derived from different electron pair arrangements often deviate from the ideal angles predicted by the VSEPR theory due to the presence of lone pairs. Here are some examples:
- Linear (2 bonding pairs, 0 lone pairs): Identical to the electron geometry. Example: BeCl₂
- Trigonal Planar (3 bonding pairs, 0 lone pairs): Identical to the electron geometry. Example: BF₃
- Bent (2 bonding pairs, 1 or 2 lone pairs): The bond angle is less than 120° due to lone pair repulsion. Example: SO₂, H₂O
- Tetrahedral (4 bonding pairs, 0 lone pairs): Identical to the electron geometry. Example: CH₄
- Trigonal Pyramidal (3 bonding pairs, 1 lone pair): The lone pair pushes the bonding pairs closer together, resulting in a pyramidal shape. Example: NH₃
- Bent (2 bonding pairs, 2 lone pairs): Similar to water, the lone pairs cause a significant compression of the bond angle. Example: H₂O
- See-saw (4 bonding pairs, 1 lone pair): Derived from a trigonal bipyramidal electron geometry. Example: SF₄
- T-shaped (3 bonding pairs, 2 lone pairs): Also derived from a trigonal bipyramidal electron geometry. Example: ClF₃
- Linear (2 bonding pairs, 3 lone pairs): Derived from a trigonal bipyramidal electron geometry. Example: XeF₂
- Square Pyramidal (5 bonding pairs, 1 lone pair): Derived from an octahedral electron geometry. Example: BrF₅
- Square Planar (4 bonding pairs, 2 lone pairs): Derived from an octahedral electron geometry. Example: XeF₄
Illustrative Examples: Highlighting the Differences
Let's examine a few examples to solidify the distinction between electron and molecular geometries:
1. Water (H₂O):
- Electron Geometry: Tetrahedral (four electron pairs around the oxygen atom: two bonding pairs and two lone pairs).
- Molecular Geometry: Bent (the two hydrogen atoms are bonded to the oxygen, forming a bent shape due to the repulsion of the two lone pairs).
2. Ammonia (NH₃):
- Electron Geometry: Tetrahedral (four electron pairs around the nitrogen atom: three bonding pairs and one lone pair).
- Molecular Geometry: Trigonal Pyramidal (the three hydrogen atoms are bonded to the nitrogen, forming a pyramidal shape with the nitrogen at the apex due to the lone pair's repulsion).
3. Methane (CH₄):
- Electron Geometry: Tetrahedral (four bonding pairs around the carbon atom).
- Molecular Geometry: Tetrahedral (the four hydrogen atoms are bonded to the carbon, forming a perfectly symmetrical tetrahedral shape; no lone pairs to distort the geometry).
4. Carbon Dioxide (CO₂):
- Electron Geometry: Linear (two electron pairs, both bonding, around the carbon atom).
- Molecular Geometry: Linear (the two oxygen atoms are bonded to the carbon in a straight line).
The Importance of Distinguishing Between Molecular and Electron Geometry
Understanding the difference between electron and molecular geometry is critical for several reasons:
- Predicting Molecular Polarity: Molecular polarity, the uneven distribution of charge within a molecule, is heavily influenced by the molecular geometry and the presence of polar bonds. Lone pairs can significantly affect the overall dipole moment.
- Explaining Physical Properties: Molecular geometry significantly influences properties like boiling point, melting point, and solubility. Molecules with similar molecular weights but different geometries can have drastically different physical properties.
- Understanding Reactivity: The shape and arrangement of atoms determine which parts of a molecule are accessible for reactions. This is crucial for predicting reactivity and designing chemical reactions.
- Spectroscopic Analysis: Molecular geometry is crucial for interpreting spectroscopic data like infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. These techniques rely on the understanding of molecular structure and bond vibrations.
Advanced Considerations and Limitations of VSEPR
While VSEPR theory is a powerful tool for predicting molecular geometries, it has limitations:
- It's a simplified model: It doesn't account for the complexities of electron interactions in larger, more complex molecules.
- It struggles with molecules containing multiple bonds: The effect of multiple bonds (double and triple bonds) on geometry isn't always accurately predicted by the basic VSEPR model.
- It doesn't predict bond lengths or angles precisely: While it predicts the overall geometry, it doesn't provide exact bond lengths or angles. More sophisticated computational methods are required for such precision.
Despite these limitations, VSEPR remains a valuable tool for visualizing and understanding the basic three-dimensional structures of molecules. Its simplicity allows for a quick estimation of molecular geometry, which is crucial for an initial understanding of chemical behavior. Combining VSEPR predictions with other computational techniques provides a more comprehensive understanding of molecular structure and properties.
Conclusion: A Unified Perspective
In summary, while both electron geometry and molecular geometry describe the spatial arrangement of atoms and electron pairs in a molecule, they offer different perspectives. Electron geometry considers all electron pairs, providing a complete picture of electron distribution. Molecular geometry, on the other hand, focuses solely on the positions of atoms, providing insight into the molecule's overall shape. Understanding both concepts, along with their differences and limitations, is essential for comprehending the properties and reactivity of molecules. The interplay between these two geometrical aspects is fundamental to a comprehensive understanding of chemical bonding and molecular behavior. By mastering these concepts, you'll enhance your understanding of chemistry and be better equipped to tackle more complex chemical concepts.
Latest Posts
Latest Posts
-
Which Of The Following Are Found In Prokaryotic Cells
Mar 31, 2025
-
Is The Square Root Of 36 Rational
Mar 31, 2025
-
Is Condensation A Chemical Or Physical Change
Mar 31, 2025
-
Is Calcium A Metal Nonmetal Or Metalloid
Mar 31, 2025
-
Adjectives That Start With The Letter C
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Molecular And Electron Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
