What Is The Difference Between Mass And Matter
Juapaving
Mar 22, 2025 · 6 min read
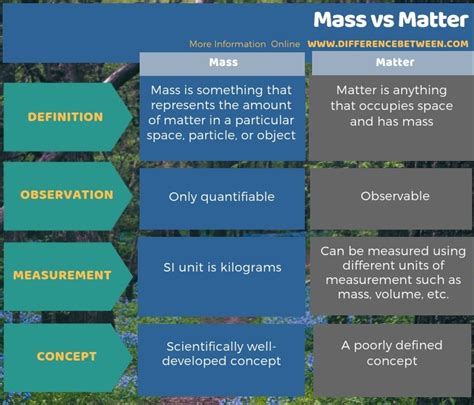
Table of Contents
What's the Difference Between Mass and Matter? A Deep Dive
The terms "mass" and "matter" are often used interchangeably in casual conversation, leading to confusion. While closely related, they represent distinct concepts in physics. Understanding the difference is crucial for grasping fundamental principles in mechanics, thermodynamics, and even cosmology. This article will delve deep into the nuances of mass and matter, exploring their definitions, relationships, and the subtle distinctions that set them apart.
Defining Matter: The Stuff of the Universe
Matter, at its most basic, is anything that occupies space and has mass. This simple definition encompasses a vast array of things, from the smallest subatomic particles to the largest celestial bodies. It's the "stuff" that makes up everything we can see, touch, and interact with in the universe.
The Building Blocks of Matter:
Matter is composed of fundamental particles called atoms. Atoms, in turn, are made up of even smaller particles:
- Protons: Positively charged particles found in the atom's nucleus.
- Neutrons: Neutral particles (no charge) also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus.
The arrangement and number of these subatomic particles determine the properties of an atom and, consequently, the properties of the matter it forms. Different combinations of protons, neutrons, and electrons create different elements (like hydrogen, oxygen, carbon, etc.), which then combine to form molecules and ultimately, all the diverse materials we encounter daily.
States of Matter:
Matter exists in various states, the most common being:
- Solid: Characterized by a fixed shape and volume. The particles are tightly packed and have strong intermolecular forces.
- Liquid: Has a fixed volume but takes the shape of its container. Particles are less tightly packed than in solids and can move more freely.
- Gas: Has neither a fixed shape nor volume. Particles are widely dispersed and move independently with high kinetic energy.
- Plasma: A highly energized state of matter where electrons are stripped from atoms, forming ions. This state is common in stars and other high-energy environments.
Understanding these states helps us comprehend the different behaviors and interactions of matter under varying conditions of temperature and pressure.
Defining Mass: A Measure of Inertia and Gravity
Mass, unlike matter, is a property of matter. It quantifies the amount of matter present and is a fundamental measure in physics. Mass is essentially a measure of two key properties:
-
Inertia: This is the resistance of an object to changes in its state of motion. A more massive object requires more force to accelerate it to the same degree as a less massive object. Think of pushing a shopping cart versus pushing a loaded truck – the truck has greater inertia.
-
Gravitational Attraction: Mass is also the source of gravitational attraction. The more massive an object, the stronger its gravitational pull on other objects. This is why planets orbit stars, and objects fall to the ground.
Different Types of Mass:
While the concept of mass might seem straightforward, there are subtle distinctions to consider:
-
Inertial Mass: This refers to the resistance of an object to acceleration. It’s measured by how much force is needed to change the object's velocity.
-
Gravitational Mass: This refers to the strength of an object's gravitational interaction with other objects. It’s measured by the force of gravity acting on the object.
Einstein's theory of general relativity equates inertial and gravitational mass, suggesting they are fundamentally the same thing. This equivalence is a cornerstone of his theory, linking space, time, and gravity.
The Relationship Between Mass and Matter:
The relationship between mass and matter is fundamental: mass is a property that quantifies the amount of matter. However, it's not a simple one-to-one correspondence. The mass of an object isn't just the sum of the masses of its constituent particles. Energy also contributes to an object's mass, as famously described by Einstein's equation: E=mc².
This equation shows that energy (E) and mass (m) are equivalent and interchangeable, with the speed of light (c) as the conversion factor. Even a small amount of energy corresponds to a tiny amount of mass, and vice versa. This is evident in nuclear reactions where a small amount of mass is converted into a vast amount of energy.
Beyond the Basics: Exploring Advanced Concepts
To fully understand the distinctions between mass and matter, we need to consider more advanced concepts:
Relativistic Mass:
In Einstein's theory of special relativity, the concept of relativistic mass arises. At very high speeds, approaching the speed of light, the mass of an object appears to increase from the perspective of a stationary observer. This is not an increase in the amount of matter, but rather a consequence of the object's increased energy. This relativistic mass is rarely used in modern physics, with the preferred term being "rest mass," which refers to the mass of an object at rest.
Dark Matter:
One of the most intriguing mysteries in cosmology is the existence of dark matter. Dark matter is a hypothetical form of matter that does not interact with light or electromagnetic radiation. We can't see it directly, but its gravitational effects are evident in the rotation of galaxies and the distribution of matter in the universe. Dark matter significantly contributes to the overall mass of the universe but its nature remains a subject of intense research.
Antimatter:
Antimatter is composed of antiparticles, which have the same mass as their corresponding particles but opposite charge. When a particle and its antiparticle collide, they annihilate each other, converting their mass entirely into energy. Antimatter is a fascinating example of matter with mass but different properties.
Mass vs. Weight: A Crucial Distinction
It's also vital to distinguish mass from weight. While related, they are not the same thing:
-
Mass: An intrinsic property of matter, independent of location. It remains constant regardless of gravitational pull.
-
Weight: The force of gravity acting on an object's mass. Weight depends on both the object's mass and the strength of the gravitational field it experiences. An object will weigh less on the moon than on Earth because the moon's gravity is weaker.
Conclusion: A nuanced understanding
In conclusion, while the terms "mass" and "matter" are often conflated, understanding their distinct meanings is crucial. Matter is the physical substance of the universe, encompassing everything that occupies space and possesses mass. Mass, on the other hand, is a quantitative property of matter, representing its resistance to acceleration and its gravitational influence. The relationship between them is intricate, involving Einstein's famous equation and more advanced concepts like relativistic mass, dark matter, and antimatter. Distinguishing mass from weight is equally important to avoid common misunderstandings in physics. By grasping these nuances, we gain a deeper appreciation for the fundamental building blocks of our universe and the forces that govern its behavior.
Latest Posts
Latest Posts
-
What Is The Prime Factors Of 24
Mar 23, 2025
-
Do Acids Turn Litmus Paper Red
Mar 23, 2025
-
A Switch Is Used In A Circuit To
Mar 23, 2025
-
What Is The Purpose Of The Commutator
Mar 23, 2025
-
What Type Of Mixture Is A Fruit Salad
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Mass And Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
