Do Acids Turn Litmus Paper Red
Juapaving
Mar 23, 2025 · 6 min read
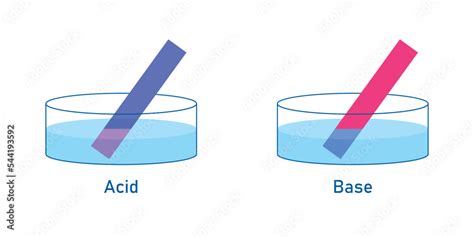
Table of Contents
Do Acids Turn Litmus Paper Red? A Deep Dive into Acid-Base Chemistry
Acids and bases are fundamental concepts in chemistry, impacting numerous aspects of our daily lives, from digestion to industrial processes. Understanding their properties, particularly their interaction with indicators like litmus paper, is crucial for grasping their significance. This comprehensive guide delves into the question: "Do acids turn litmus paper red?" exploring the underlying chemistry, the mechanism of litmus paper, and the broader implications of acid-base reactions.
Understanding Acids and Bases
Before we explore the interaction between acids and litmus paper, let's establish a clear understanding of what acids and bases are. Several definitions exist, but the most commonly used are the Arrhenius and Brønsted-Lowry definitions.
Arrhenius Definition
The Arrhenius definition, proposed by Svante Arrhenius in 1884, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻) when dissolved in water. This definition, while simple, has limitations as it only applies to aqueous solutions.
Examples:
- Acid: Hydrochloric acid (HCl) dissociates in water to form H⁺ and Cl⁻ ions.
- Base: Sodium hydroxide (NaOH) dissociates in water to form Na⁺ and OH⁻ ions.
Brønsted-Lowry Definition
The Brønsted-Lowry definition, proposed by Johannes Nicolaus Brønsted and Thomas Martin Lowry independently in 1923, provides a broader perspective. It defines acids as proton donors and bases as proton acceptors. This definition encompasses a wider range of substances, including those that don't necessarily involve hydroxide ions.
Examples:
- Acid: HCl donates a proton (H⁺) to water, forming H₃O⁺ (hydronium ion) and Cl⁻.
- Base: Ammonia (NH₃) accepts a proton from water, forming NH₄⁺ (ammonium ion) and OH⁻.
This broader definition is more comprehensive and explains acid-base reactions in a wider variety of solvents beyond just water.
The Role of pH and the pH Scale
The acidity or basicity of a solution is quantified using the pH scale. The pH scale is a logarithmic scale ranging from 0 to 14, where:
- pH 0-7: Indicates an acidic solution. The lower the pH, the stronger the acid.
- pH 7: Indicates a neutral solution (like pure water).
- pH 7-14: Indicates a basic (or alkaline) solution. The higher the pH, the stronger the base.
The pH of a solution is determined by the concentration of hydrogen ions (H⁺). A lower concentration of H⁺ means a higher pH (more basic), and a higher concentration of H⁺ means a lower pH (more acidic).
Litmus Paper: A Natural Acid-Base Indicator
Litmus paper is a crucial tool in acid-base chemistry. It's a type of indicator, a substance that changes color depending on the pH of the solution it's in contact with. Litmus paper is derived from lichens, which contain various pigments sensitive to changes in pH.
How Litmus Paper Works
The color change in litmus paper is due to the presence of different chemical compounds within the lichen extract. These compounds undergo structural changes upon exposure to H⁺ or OH⁻ ions, resulting in a visible color shift. In acidic solutions, these compounds change their structure, absorbing different wavelengths of light, leading to a color change.
The Color Change: Red and Blue Litmus Paper
Litmus paper comes in two forms: red and blue.
- Red litmus paper: Turns blue in the presence of a base (alkaline solution).
- Blue litmus paper: Turns red in the presence of an acid.
Therefore, to directly answer the question, yes, acids turn blue litmus paper red. This color change is a clear indication that the solution being tested is acidic.
Beyond Litmus Paper: Other Acid-Base Indicators
While litmus paper is a convenient and readily available indicator, other indicators provide a more precise determination of pH. These include:
- Phenolphthalein: Colorless in acidic solutions and pink in basic solutions.
- Methyl orange: Red in acidic solutions and yellow in basic solutions.
- Bromothymol blue: Yellow in acidic solutions, green in neutral solutions, and blue in basic solutions.
These indicators have different pH ranges over which they change color, offering more nuanced information about the acidity or basicity of a solution. The choice of indicator depends on the specific pH range being investigated.
Applications of Acid-Base Indicators
Acid-base indicators have wide-ranging applications across various fields:
- Chemistry Labs: Essential tools for determining the pH of solutions during titrations and other experiments.
- Environmental Monitoring: Used to assess the acidity of water samples, crucial for monitoring water quality and pollution levels.
- Medicine: Used in certain diagnostic tests and monitoring of bodily fluids.
- Food Industry: Used in the production and quality control of food products.
The Chemical Mechanism Behind the Color Change
The color change observed in litmus paper and other indicators is a result of changes in the molecular structure of the indicator molecule. These changes are triggered by the presence of H⁺ or OH⁻ ions. The indicator molecules act as weak acids or bases themselves. When they interact with a strong acid or base, a proton transfer occurs, altering the electron distribution in the molecule and thus its ability to absorb and reflect different wavelengths of light. This leads to the observable color change. This process is often referred to as a tautomerization, a structural isomerism involving a proton shift. The different tautomers have distinct color properties.
Practical Applications and Considerations
When using litmus paper, it's crucial to consider a few important points:
- Proper Technique: Dip a clean, dry strip of litmus paper into the solution to be tested. Avoid contaminating the solution or the litmus paper.
- Interpretation: Observe the color change carefully, comparing it to the original color of the litmus paper.
- Limitations: Litmus paper provides a qualitative indication of acidity or basicity but doesn’t provide a precise pH value. For precise pH measurements, a pH meter is necessary.
- Storage: Store litmus paper in a dry, airtight container to prevent its degradation and maintain its accuracy.
Conclusion: Acids, Bases, and the Significance of Indicators
The question, "Do acids turn litmus paper red?" leads us on a fascinating journey into the world of acid-base chemistry. The answer, a resounding yes (for blue litmus paper), highlights the importance of indicators like litmus paper in identifying and understanding the properties of acidic and basic solutions. While litmus paper offers a simple and qualitative assessment, it serves as a gateway to a deeper appreciation of the complex interactions between acids, bases, and the indicators that reveal their nature. The principles involved extend far beyond the simple color change, impacting a wide range of scientific fields and practical applications. Understanding these fundamental concepts is crucial for anyone seeking a deeper understanding of chemistry and its applications in the world around us. Beyond litmus paper, the various indicators and the understanding of pH measurement offer powerful tools for various scientific and industrial applications, emphasizing the continued relevance and significance of acid-base chemistry.
Latest Posts
Latest Posts
-
How Many 1 5 Liters In A Gallon
Mar 25, 2025
-
84 As A Product Of Prime Factors
Mar 25, 2025
-
Least Common Multiple Of 4 6 8
Mar 25, 2025
-
In A Bacterium Where Are Proteins Synthesized
Mar 25, 2025
-
How Many Valence Electrons In Bromine
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Do Acids Turn Litmus Paper Red . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
