What Is The Difference Between Electron And Molecular Geometry
Juapaving
Mar 30, 2025 · 6 min read
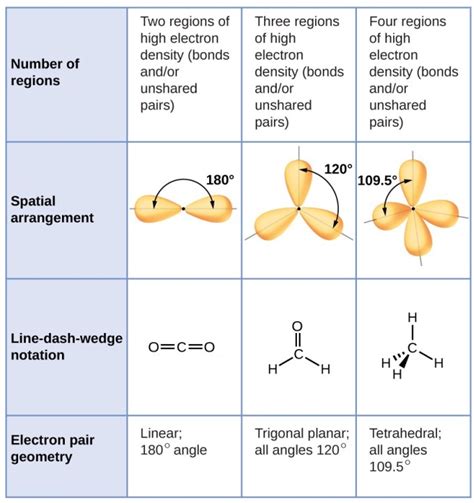
Table of Contents
What's the Difference Between Electron and Molecular Geometry? A Deep Dive
Understanding the shapes of molecules is fundamental to chemistry. It dictates their reactivity, physical properties, and biological functions. However, the terms "electron geometry" and "molecular geometry" are often confused. While closely related, they represent different aspects of a molecule's three-dimensional structure. This article will delve into the core differences between these concepts, providing clear explanations, illustrative examples, and practical tips to master this crucial topic.
Electron Geometry: The Complete Picture
Electron geometry describes the arrangement of all electron domains around the central atom in a molecule. An electron domain is simply a region of space where electrons are likely to be found. This includes both bonding pairs (electrons shared between atoms in a covalent bond) and lone pairs (electrons not involved in bonding). The key is that electron geometry considers all electrons around the central atom, regardless of whether they are bonding or non-bonding.
VSEPR Theory: The Guiding Principle
The Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone of understanding electron geometry. VSEPR theory postulates that electron domains repel each other and will arrange themselves to be as far apart as possible to minimize repulsion. This leads to predictable geometric shapes depending on the number of electron domains around the central atom.
Common Electron Geometries
Let's explore some of the most common electron geometries:
- Linear: Two electron domains arranged 180° apart. Example: BeCl₂
- Trigonal Planar: Three electron domains arranged 120° apart in a plane. Example: BF₃
- Tetrahedral: Four electron domains arranged 109.5° apart in a three-dimensional tetrahedron. Example: CH₄
- Trigonal Bipyramidal: Five electron domains arranged in a trigonal bipyramidal shape. This geometry features two different bond angles: 90° and 120°. Example: PCl₅
- Octahedral: Six electron domains arranged 90° apart in an octahedral shape. Example: SF₆
Visualizing Electron Geometry: The Importance of Lone Pairs
It's crucial to remember that lone pairs are also electron domains and contribute to the overall electron geometry. While they don't directly participate in bonding, they significantly influence the molecule's shape by repelling bonding pairs. A molecule with lone pairs will have a different molecular geometry, even if its electron geometry is the same as a molecule without lone pairs.
Molecular Geometry: The Shape of the Molecule
Molecular geometry, also known as molecular shape, focuses solely on the arrangement of atoms within a molecule. Unlike electron geometry, it only considers the positions of the atoms bonded to the central atom. Lone pairs, while influencing the overall shape by their repulsive forces, are not included in the description of molecular geometry.
Distinguishing Electron and Molecular Geometry
The critical difference lies in the inclusion or exclusion of lone pairs. Electron geometry considers all electron domains, including lone pairs, whereas molecular geometry considers only the atoms bonded to the central atom. This leads to situations where a molecule has the same electron geometry but a different molecular geometry due to varying numbers of lone pairs.
Common Molecular Geometries
Several molecular geometries correspond to different electron geometries, reflecting the influence of lone pairs:
- Linear: Two bonding pairs, no lone pairs. Example: BeCl₂ (Both electron and molecular geometry are linear)
- Trigonal Planar: Three bonding pairs, no lone pairs. Example: BF₃ (Both electron and molecular geometry are trigonal planar)
- Bent (V-shaped): Three electron domains (two bonding pairs and one lone pair). Example: H₂O (Electron geometry is trigonal planar; molecular geometry is bent)
- Tetrahedral: Four bonding pairs, no lone pairs. Example: CH₄ (Both electron and molecular geometry are tetrahedral)
- Trigonal Pyramidal: Four electron domains (three bonding pairs and one lone pair). Example: NH₃ (Electron geometry is tetrahedral; molecular geometry is trigonal pyramidal)
- Bent (V-shaped): Four electron domains (two bonding pairs and two lone pairs). Example: H₂S (Electron geometry is tetrahedral; molecular geometry is bent)
- See-Saw: Five electron domains (four bonding pairs and one lone pair). Example: SF₄ (Electron geometry is trigonal bipyramidal; molecular geometry is see-saw)
- T-shaped: Five electron domains (three bonding pairs and two lone pairs). Example: ClF₃ (Electron geometry is trigonal bipyramidal; molecular geometry is T-shaped)
- Linear: Five electron domains (two bonding pairs and three lone pairs). Example: XeF₂ (Electron geometry is trigonal bipyramidal; molecular geometry is linear)
- Square Pyramidal: Six electron domains (five bonding pairs and one lone pair). Example: BrF₅ (Electron geometry is octahedral; molecular geometry is square pyramidal)
- Square Planar: Six electron domains (four bonding pairs and two lone pairs). Example: XeF₄ (Electron geometry is octahedral; molecular geometry is square planar)
Predicting Geometries: A Step-by-Step Approach
Predicting the electron and molecular geometries involves a systematic process:
-
Draw the Lewis Structure: This shows the arrangement of atoms and electrons in the molecule. Determine the central atom and count the number of valence electrons.
-
Count Electron Domains: This includes both bonding pairs and lone pairs around the central atom.
-
Determine Electron Geometry: Use VSEPR theory to predict the arrangement of electron domains based on the number of domains (linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral).
-
Determine Molecular Geometry: Consider only the positions of the atoms bonded to the central atom, ignoring lone pairs. Use the appropriate terminology based on the arrangement of atoms (linear, bent, trigonal pyramidal, tetrahedral, see-saw, T-shaped, square pyramidal, square planar).
The Impact of Geometry on Molecular Properties
The geometry of a molecule profoundly impacts its properties:
-
Polarity: Molecular polarity is determined by the arrangement of polar bonds and the overall molecular symmetry. Symmetrical molecules, such as CO₂, can have nonpolar overall polarity, even with polar bonds, while asymmetrical molecules, like H₂O, will be polar.
-
Reactivity: The accessibility of atoms and the spatial orientation of bonds affect the molecule's reactivity. Steric hindrance, the obstruction of reactions due to spatial limitations, is directly linked to molecular geometry.
-
Boiling Point and Melting Point: Intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, are strongly influenced by molecular shape. These forces affect the boiling and melting points of substances.
-
Solubility: The solubility of a molecule depends on its ability to interact with solvent molecules. Molecular shape plays a crucial role in determining the strength and type of these interactions.
Beyond Simple Molecules: Complex Cases
While the principles outlined here apply to many molecules, some complex molecules may exhibit deviations from the idealized geometries predicted by VSEPR theory. Factors such as multiple bonds, steric effects, and the presence of large substituents can influence the actual molecular shape.
Conclusion: Mastering the Nuances
Understanding the difference between electron and molecular geometry is paramount for grasping the intricacies of molecular structure and its relationship to chemical and physical properties. By systematically applying VSEPR theory and considering the influence of lone pairs, one can accurately predict and understand the three-dimensional arrangement of atoms and electrons within molecules. This knowledge provides a foundation for comprehending a wide range of chemical phenomena and behaviors. Remember to always visualize the molecule to strengthen your understanding; practice makes perfect in this area of chemistry. With consistent effort, you can master these concepts and confidently tackle more complex molecular structures.
Latest Posts
Latest Posts
-
Least Common Multiple For 9 And 12
Apr 01, 2025
-
Examples Of The Eight Parts Of Speech
Apr 01, 2025
-
Is Anything That Occupies Space And Has Mass
Apr 01, 2025
-
Mario Has To Take Medication For 180 Days
Apr 01, 2025
-
What Is On A Physical Map
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Electron And Molecular Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
