What Is The Difference Between A Solution And A Mixture
Juapaving
Mar 22, 2025 · 6 min read
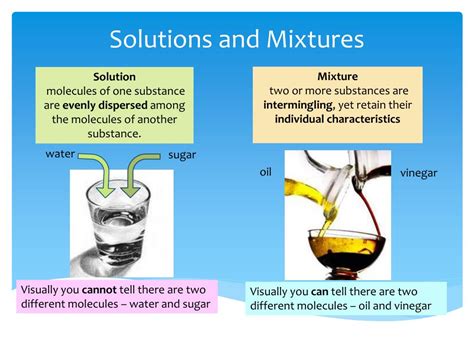
Table of Contents
What's the Difference Between a Solution and a Mixture? A Deep Dive into Chemistry
Understanding the difference between a solution and a mixture is fundamental to grasping many chemical concepts. While both involve combining different substances, the key lies in the level of homogeneity and the nature of the interaction between the components. This article delves deep into the distinctions, exploring examples, properties, and applications to solidify your understanding.
Solutions: A Uniform Blend at the Molecular Level
A solution is a homogeneous mixture composed of two or more substances. The crucial aspect here is homogeneity: the components are uniformly distributed throughout the mixture at a molecular level. You won't be able to visually distinguish the individual components; they're thoroughly integrated.
Key Characteristics of Solutions:
- Homogeneity: This is the defining feature. No matter where you sample a solution, its composition will be identical. Think of saltwater: a tiny drop will have the same salt-to-water ratio as a large bucketful.
- Particle Size: The solute particles (the substance being dissolved) are extremely small, typically at the atomic or molecular level. This microscopic size contributes to the solution's transparency (unless the solution itself absorbs light).
- Solubility: The ability of a solute to dissolve in a solvent (the substance doing the dissolving) is crucial. Solubility depends on factors like temperature, pressure, and the chemical properties of the solute and solvent.
- Filtration: Solutions cannot be separated by simple filtration. The solute particles are too small to be trapped by filter paper.
- Examples: Saltwater (salt dissolved in water), sugar dissolved in water, air (a mixture of gases), many alloys (like brass – a mixture of copper and zinc).
Types of Solutions:
Solutions can be classified based on the state of matter of the solute and solvent:
- Gaseous solutions: Air is a classic example, with various gases dissolved in each other.
- Liquid solutions: This is the most common type. Examples include saltwater, sugar water, and many alcoholic beverages.
- Solid solutions: Alloys are excellent examples, where one metal is dissolved in another.
Mixtures: A Broader Category with Variable Composition
Mixtures, on the other hand, are a much broader category. They encompass a combination of two or more substances where the individual components retain their chemical identities. Unlike solutions, mixtures don't necessarily have a uniform composition.
Key Characteristics of Mixtures:
- Heterogeneity (often): Many mixtures are heterogeneous, meaning the components are not uniformly distributed. You can often visually distinguish the different parts.
- Variable Composition: The ratio of components in a mixture can vary widely.
- Retention of Properties: The individual components retain their unique chemical and physical properties.
- Separation: The components of a mixture can often be separated by physical methods such as filtration, decantation, distillation, or evaporation.
- Examples: Sand and water, oil and water, a salad, granite (a mixture of minerals).
Types of Mixtures:
Mixtures are broadly classified into two main types:
- Homogeneous Mixtures: While we've established that solutions are a type of homogeneous mixture, there are other examples that aren't solutions. Air, for instance, is a homogeneous mixture of gases, but it's not a solution in the strict chemical sense because gases don't typically "dissolve" in each other in the same way a solid dissolves in a liquid. Similarly, some alloys behave more like homogeneous mixtures than true solutions.
- Heterogeneous Mixtures: These mixtures have a non-uniform composition. You can easily see the different components. Examples range from simple things like sand and water to more complex materials like concrete.
The Subtleties and Overlaps: Where the Lines Blur
The distinction between solutions and mixtures isn't always crystal clear. Some mixtures exhibit properties that fall somewhere between a truly homogeneous solution and a distinctly heterogeneous mixture. This is especially true with colloids and suspensions.
Colloids: A Gray Area
Colloids are a fascinating middle ground. They appear homogeneous to the naked eye, but under magnification, you'd see that the dispersed particles are larger than those in a solution. These particles are typically in the nanometer to micrometer range. Because of this intermediate particle size, they don't settle out easily like suspensions.
Examples of Colloids:
- Milk: Fat globules dispersed in water.
- Fog: Water droplets dispersed in air.
- Mayonnaise: Oil droplets dispersed in water (stabilized by an emulsifier).
Suspensions: Distinctly Heterogeneous
Suspensions are heterogeneous mixtures where the dispersed particles are much larger than in solutions or colloids. These particles will eventually settle out if left undisturbed.
Examples of Suspensions:
- Muddy water: Soil particles suspended in water.
- Blood: Blood cells suspended in plasma.
- Paint: Pigment particles suspended in a liquid medium.
Table Summarizing the Key Differences:
| Feature | Solution | Mixture | Colloid | Suspension |
|---|---|---|---|---|
| Homogeneity | Homogeneous | Often heterogeneous, can be homogeneous | Appears homogeneous, but isn't | Heterogeneous |
| Particle Size | Atomic/molecular | Variable, larger than in solutions | Nano to micrometer | Micrometer to larger |
| Settlement | Does not settle | May settle (heterogeneous mixtures) | Does not readily settle | Settles readily |
| Filtration | Cannot be separated by filtration | Can often be separated by filtration | Cannot be separated by simple filtration | Can be separated by filtration |
| Examples | Saltwater, air, alloys | Sand and water, oil and water, salad | Milk, fog, mayonnaise | Muddy water, blood, paint |
Practical Applications and Importance
Understanding the differences between solutions and mixtures is crucial in various fields:
- Chemistry: It forms the basis of understanding reaction rates, solubility, and other chemical processes.
- Materials Science: The properties of materials are often directly linked to whether they are solutions, mixtures, colloids, or suspensions. This understanding is critical in designing new materials with specific properties.
- Environmental Science: Understanding how pollutants behave in solutions or as suspensions in water or air is crucial for environmental monitoring and remediation.
- Medicine: Many drugs are administered as solutions or suspensions. The formulation affects how the drug is absorbed and its efficacy.
- Food Science: Food products often involve solutions, mixtures, colloids, and suspensions. Understanding these aspects is crucial for food processing, stability, and texture.
Conclusion: A Foundation for Deeper Understanding
The distinction between a solution and a mixture is a cornerstone of chemistry. While the definition of a solution might seem straightforward, the nuances—particularly with colloids and suspensions—highlight the complexity of matter at the molecular and macroscopic levels. By understanding the fundamental differences between these types of matter, you unlock a deeper appreciation for the vast world of chemical interactions and their diverse applications. This knowledge is not merely academic; it's essential for advancing scientific knowledge and solving real-world problems across various disciplines.
Latest Posts
Latest Posts
-
What Is The Mass Of 1 Mole Of Water
Mar 22, 2025
-
Which Of The Following Is The Correct Accounting Equation
Mar 22, 2025
-
What Two Organelles Are Found Only In Plant Cells
Mar 22, 2025
-
Write An Inverse Variation Equation That Relates X And Y
Mar 22, 2025
-
How Many Turns Of The Krebs Cycle Per Glucose
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Solution And A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
