What Is The Charge Of Beryllium
Juapaving
Mar 27, 2025 · 5 min read
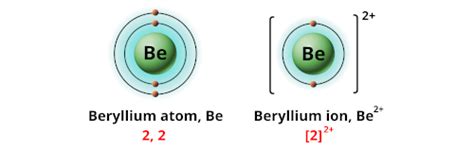
Table of Contents
What is the Charge of Beryllium? Understanding Beryllium's Oxidation States and Chemical Behavior
Beryllium, a fascinating and relatively rare alkaline earth metal, holds a unique position in the periodic table. Its properties, particularly its charge and how it interacts chemically, are crucial for understanding its applications in various fields, from aerospace engineering to nuclear technology. This comprehensive article delves deep into the charge of beryllium, exploring its oxidation states, chemical bonding, and the implications of its unique electronic configuration.
Understanding Beryllium's Electronic Configuration
To grasp the charge of beryllium, we must first understand its electronic structure. Beryllium (Be) has an atomic number of 4, meaning it possesses four protons and, in its neutral state, four electrons. These electrons are arranged in two electron shells: two electrons occupy the 1s orbital, and the remaining two reside in the 2s orbital. This configuration is represented as 1s²2s².
This electronic arrangement dictates beryllium's chemical behavior and its tendency to lose electrons to achieve a stable electron configuration. The outermost shell, containing two electrons, is the valence shell. These valence electrons are the key players in determining the charge beryllium will exhibit in chemical compounds.
Beryllium's Most Common Oxidation State: +2
Beryllium almost exclusively exhibits a +2 oxidation state. This means it readily loses its two valence electrons to achieve the stable electron configuration of helium (1s²), a noble gas with a filled electron shell. This electron loss results in a beryllium ion, denoted as Be²⁺. This is the dominant charge observed in virtually all beryllium compounds.
Why +2? The Octet Rule and Stability
The driving force behind beryllium's +2 oxidation state is the octet rule, a fundamental principle in chemistry. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. While beryllium doesn't achieve an octet in the strictest sense by losing two electrons (it only has two shells, and the inner shell is already full), it achieves a stable noble gas configuration, mimicking helium's stability. This is energetically favorable and a significant factor in its chemical reactivity.
Rare Instances of Other Oxidation States? A Deeper Look
While +2 is the overwhelmingly prevalent oxidation state, there has been some theoretical and limited experimental investigation into the possibility of other oxidation states for beryllium. These possibilities are largely hypothetical and under intense scrutiny.
Challenges in Observing Other Oxidation States
The high ionization energies required to remove more than two electrons from beryllium make higher oxidation states extremely unlikely under normal conditions. The energy required to strip away additional electrons is significantly greater than the energy released by forming bonds, rendering these states highly unstable.
Computational Studies and Predictions
Computational chemistry, using advanced quantum mechanical calculations, has attempted to predict the existence of higher oxidation states, such as +4. These studies suggest the possibility of such states under very specific, extreme conditions, such as those found in high-energy environments or in reactions with highly electronegative atoms. However, these theoretical predictions haven't yet been conclusively confirmed experimentally.
Beryllium's Chemical Bonding: Ionic and Covalent Aspects
Beryllium's +2 charge significantly influences the type of chemical bonds it forms. While it predominantly participates in ionic bonding (transferring electrons), it also displays a significant degree of covalent bonding (sharing electrons), especially when bonding with highly electronegative atoms such as oxygen and fluorine.
Ionic Bonding in Beryllium Compounds
In ionic compounds, beryllium readily loses its two valence electrons to form Be²⁺ ions, which are then electrostatically attracted to negatively charged anions (ions with an excess of electrons). For instance, in beryllium oxide (BeO), beryllium loses two electrons to oxygen atoms, forming a crystalline solid held together by strong electrostatic forces.
Covalent Character in Beryllium Bonds: Polarization and Electronegativity
While ionic bonding dominates, the high charge density of the Be²⁺ ion leads to significant polarization of the electron cloud in its covalent bonds. This polarization results in a substantial covalent character in bonds involving beryllium, particularly with highly electronegative atoms. The electronegativity difference between beryllium and the other atom determines the degree of ionic versus covalent character in the bond.
The Importance of Beryllium's Charge in Its Applications
Beryllium's unique charge and resulting properties are crucial to its diverse applications. Its light weight, high strength-to-weight ratio, and excellent thermal conductivity, all stemming from its electronic structure and bonding characteristics, make it a valuable material in several industries:
Aerospace Industry: Lightweight and High-Strength Materials
Beryllium's low density and high strength make it ideal for aerospace applications, where reducing weight is crucial for fuel efficiency and maneuverability. It's used in aircraft parts, spacecraft components, and missiles.
Nuclear Technology: Neutron Moderator and Reflector
Beryllium's low atomic mass and low neutron absorption cross-section allow it to serve as an effective neutron moderator and reflector in nuclear reactors. This property is essential for controlling the nuclear chain reaction and improving the reactor's efficiency.
Electronics Industry: High-Performance Electronics
Beryllium's unique combination of properties makes it suitable for electronic applications. Its high thermal conductivity helps dissipate heat effectively, crucial for high-power electronic components.
Other Applications: X-ray windows, high-precision instruments
Its transparency to X-rays makes it valuable for X-ray windows. Its dimensional stability and stiffness are also utilized in creating high-precision instruments.
Safety Considerations and Toxicity
Despite its valuable properties, beryllium is a highly toxic substance. Inhalation of beryllium dust can lead to a serious lung disease called berylliosis, which can be debilitating and even fatal. Therefore, appropriate safety precautions and handling procedures are crucial when working with beryllium and its compounds.
Conclusion: Beryllium's Charge as a Key to Understanding its Behavior
The charge of beryllium, almost exclusively +2, is fundamental to understanding its chemical behavior, bonding characteristics, and a wide range of applications. While theoretical studies suggest other oxidation states might exist under extreme conditions, the +2 oxidation state dominates its chemistry and explains its unique properties. This understanding is crucial for responsible use and handling of this versatile but potentially hazardous metal. Further research into the intricacies of its chemical bonding and potential for other oxidation states continues to expand our knowledge of this fascinating element.
Latest Posts
Latest Posts
-
Least Common Denominator Of Rational Expressions Calculator
Mar 30, 2025
-
What Is The Least Common Multiple Of 25 And 30
Mar 30, 2025
-
What Is 3 8 Of A Full Rotation
Mar 30, 2025
-
Which Is Bigger 3 8 Or 1 2
Mar 30, 2025
-
What Other Organelle Besides The Nucleus Contain Dna
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Beryllium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
