What Is The Average Kinetic Energy
Juapaving
Mar 14, 2025 · 7 min read
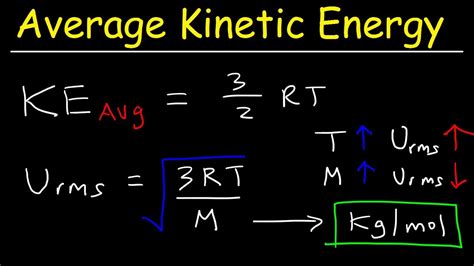
Table of Contents
What is the Average Kinetic Energy? A Deep Dive into Molecular Motion and Thermal Physics
Understanding the average kinetic energy is crucial for grasping fundamental concepts in physics, particularly in thermodynamics and statistical mechanics. It bridges the macroscopic world we observe with the microscopic world of atoms and molecules, providing a powerful tool for explaining phenomena like temperature and heat transfer. This article will delve deep into the concept of average kinetic energy, exploring its definition, calculation, applications, and implications across various scientific disciplines.
Defining Average Kinetic Energy
The kinetic energy of a single particle is defined as the energy it possesses due to its motion. For a particle of mass m moving with velocity v, its kinetic energy (KE) is given by the equation:
KE = ½mv²
However, in systems containing a vast number of particles, like a gas in a container, it's impossible to track the individual kinetic energies of each particle. Instead, we focus on the average kinetic energy of the particles. This average kinetic energy is directly related to the temperature of the system. The higher the average kinetic energy, the higher the temperature.
It's important to note that the term "average" refers to the mean kinetic energy across all the particles in the system. Individual particles will have varying kinetic energies due to random collisions and interactions. The average kinetic energy provides a statistical representation of the system's overall energy state.
Calculating Average Kinetic Energy: The Ideal Gas Law
For an ideal gas (a theoretical model that approximates the behavior of real gases under certain conditions), the average kinetic energy of the particles can be directly related to the temperature through the ideal gas law:
PV = nRT
where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of gas
- R is the ideal gas constant (8.314 J/mol·K)
- T is the absolute temperature in Kelvin
While this equation doesn't explicitly state average kinetic energy, it's intimately connected. Through the kinetic theory of gases, we can derive a relationship between the average kinetic energy and temperature:
KE<sub>avg</sub> = (3/2)kT
where:
- KE<sub>avg</sub> is the average kinetic energy per particle
- k is the Boltzmann constant (1.38 × 10⁻²³ J/K)
- T is the absolute temperature in Kelvin
This equation is a cornerstone of statistical mechanics and highlights the direct proportionality between average kinetic energy and absolute temperature. As temperature increases, the average kinetic energy of the gas particles increases proportionally.
Applications of Average Kinetic Energy
The concept of average kinetic energy finds extensive applications in various fields, including:
1. Thermodynamics: Understanding Heat and Temperature
Average kinetic energy is the fundamental link between the microscopic world of molecular motion and the macroscopic property of temperature. Heat transfer is essentially the transfer of average kinetic energy from a hotter (higher average KE) system to a colder (lower average KE) system. Understanding this connection allows us to analyze thermodynamic processes like heating, cooling, and phase transitions.
2. Statistical Mechanics: Modeling Complex Systems
Statistical mechanics employs probabilistic methods to describe the behavior of large systems containing many particles. Average kinetic energy plays a pivotal role in these models, providing a way to characterize the overall energy distribution and predict macroscopic properties based on microscopic interactions.
3. Chemical Kinetics: Reaction Rates and Activation Energy
The rate at which chemical reactions occur is directly influenced by the average kinetic energy of the reactant molecules. For a reaction to proceed, the colliding molecules must possess sufficient kinetic energy to overcome the activation energy barrier. Higher temperatures, and thus higher average kinetic energy, lead to faster reaction rates.
4. Atmospheric Science: Modeling Weather Patterns
Atmospheric models rely heavily on understanding the average kinetic energy of air molecules. This energy governs wind speed, atmospheric pressure variations, and the formation of weather systems. Changes in average kinetic energy due to solar radiation and other factors drive atmospheric dynamics.
5. Astrophysics: Stellar Evolution and Plasma Physics
In astrophysics, average kinetic energy is crucial for understanding stellar processes. The immense temperatures and pressures within stars lead to extremely high average kinetic energies of the constituent particles (primarily hydrogen and helium). These high kinetic energies drive nuclear fusion reactions, providing the energy that powers stars. In plasma physics, the study of ionized gases, average kinetic energy plays a key role in understanding plasma behavior and its interactions with magnetic fields.
Beyond Ideal Gases: Real-World Considerations
While the ideal gas law and the (3/2)kT formula provide a good approximation for many systems, real gases deviate from ideal behavior at high pressures and low temperatures. Intermolecular forces, which are neglected in the ideal gas model, become significant under these conditions, affecting the average kinetic energy. More sophisticated models, such as the van der Waals equation, are needed to account for these deviations.
Calculating Average Kinetic Energy in More Complex Systems
Calculating the average kinetic energy for systems beyond ideal gases can be significantly more complex. For liquids and solids, the interactions between particles are much stronger and more intricate than in gases. Computational methods, like molecular dynamics simulations, are often employed to determine the average kinetic energy in these systems. These simulations use powerful computers to track the motion of individual particles and calculate their kinetic energies, leading to an accurate estimation of the average kinetic energy.
The Relationship Between Average Kinetic Energy and Temperature: A Deeper Look
The direct proportionality between average kinetic energy and absolute temperature is a remarkable result, reflecting the fundamental connection between microscopic motion and macroscopic properties. This relationship allows us to define temperature as a measure of the average kinetic energy of particles in a system. However, this relationship is not universal and holds most accurately for ideal gases. In other systems, factors such as intermolecular forces and the vibrational and rotational energy of molecules contribute to the overall energy and need to be considered when defining temperature.
Average Kinetic Energy and Brownian Motion
A fascinating demonstration of average kinetic energy is Brownian motion, the random movement of microscopic particles suspended in a fluid. This motion is caused by the incessant bombardment of the particles by the surrounding fluid molecules. The magnitude of this motion is directly related to the average kinetic energy of the fluid molecules. Observations of Brownian motion provided crucial evidence for the existence of atoms and molecules and helped solidify the kinetic theory of gases.
Limitations and Refinements
It's essential to acknowledge the limitations of the simple (3/2)kT formula for average kinetic energy. This equation is derived for monatomic ideal gases, meaning gases composed of single atoms. For more complex molecules (e.g., diatomic gases like oxygen or nitrogen), rotational and vibrational degrees of freedom also contribute to the total energy, altering the relationship between average kinetic energy and temperature. The total kinetic energy will be greater, distributed across translational, rotational, and vibrational modes of motion. The equipartition theorem provides a framework for distributing energy among these various degrees of freedom, leading to more accurate predictions of average kinetic energy in more complex systems.
Conclusion: The Significance of Average Kinetic Energy
The average kinetic energy is a powerful concept that connects the microscopic world of atoms and molecules to the macroscopic world of observable properties. It is a fundamental quantity in thermodynamics, statistical mechanics, and numerous other scientific disciplines. Understanding its calculation, applications, and limitations is crucial for comprehending the behavior of matter and energy at all scales, from the smallest particles to the largest astronomical objects. While the simple (3/2)kT formula provides a good starting point, more sophisticated models and computational methods are needed for accurately describing the average kinetic energy in real-world systems of varying complexities. The ongoing research and development in this field continue to refine our understanding of this essential concept and its role in shaping the physical world around us.
Latest Posts
Latest Posts
-
Digestion Of Food Is Chemical Or Physical Change
Mar 15, 2025
-
During The Energy Investment Phase Of Glycolysis
Mar 15, 2025
-
How Many Flat Surfaces Does A Rectangular Prism Have
Mar 15, 2025
-
Lowest Common Multiple Of 9 And 8
Mar 15, 2025
-
Real Life Examples Of Right Angles
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Average Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
