What Is The Atomic Symbol For Potassium
Juapaving
Mar 25, 2025 · 7 min read
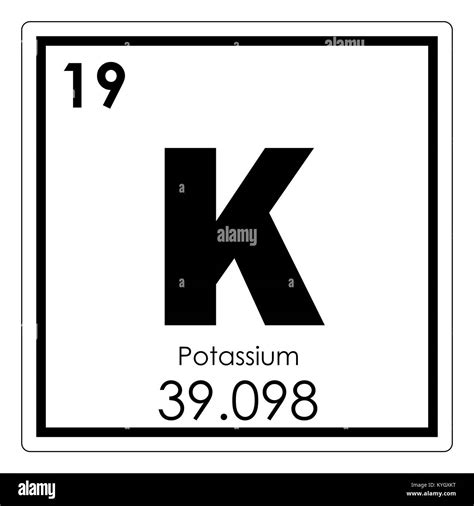
Table of Contents
What is the Atomic Symbol for Potassium? A Deep Dive into the Element
Potassium, an essential element for life, plays a crucial role in numerous biological processes. Understanding its properties, including its atomic symbol, is vital for anyone interested in chemistry, biology, or related fields. This comprehensive guide explores the atomic symbol for potassium, delving into its history, discovery, properties, and significance.
Understanding Atomic Symbols
Before diving into the specifics of potassium, let's establish a foundational understanding of atomic symbols. In chemistry, an atomic symbol is a one or two-letter abbreviation used to represent a chemical element. These symbols are derived from the element's name, either in English or its Latin equivalent. They are universally recognized and form the basis of chemical formulas and equations. The atomic symbol is a shorthand that simplifies complex chemical interactions and facilitates clear communication among scientists worldwide. For example, H represents hydrogen, O represents oxygen, and Na represents sodium (from the Latin natrium).
The usefulness of atomic symbols extends beyond mere shorthand notation. They provide a standardized way to identify elements, irrespective of the language being used. This uniformity is crucial for international scientific collaboration. Furthermore, atomic symbols are integral to understanding the structure of molecules and predicting the properties of compounds.
Potassium: The Atomic Symbol and Beyond
The atomic symbol for potassium is K. This symbol originates from the Latin word kalium, which refers to potash, a source from which potassium compounds were historically extracted. This historical connection underscores the rich history of scientific discovery and the evolution of chemical nomenclature. The use of "K" as the symbol, rather than a symbol derived directly from "potassium," reflects the long-standing tradition of using Latin-based abbreviations in chemistry.
While "K" might seem arbitrary at first, it maintains consistency within the established system of chemical notation. The adoption of Latin-derived symbols for many elements ensures a global understanding and avoids potential ambiguity caused by linguistic variations. This consistent use of symbols is fundamental to the precise communication that underpins scientific progress.
The Discovery and History of Potassium
The isolation of potassium as a pure element was a significant milestone in chemical history. It wasn't a simple discovery, but rather a gradual process involving various scientists and evolving techniques. While its compounds were known and utilized for centuries (potash in agriculture, for example), isolating the pure element required significant advancements in chemistry.
Sir Humphry Davy, a pioneering figure in electrochemistry, is credited with isolating potassium in 1807. He achieved this using electrolysis, a groundbreaking technique at the time. By passing an electric current through molten potassium hydroxide, he successfully separated potassium metal, thus marking a significant advance in elemental isolation and understanding. This discovery confirmed the existence of potassium as a distinct element and helped solidify the understanding of chemical bonding and the nature of elements.
The Properties of Potassium: A Detailed Look
Potassium is a highly reactive alkali metal. It's characterized by several key properties:
-
Appearance: Potassium is a silvery-white, soft metal. Its softness is a characteristic of alkali metals, reflecting their relatively weak metallic bonding. It can be easily cut with a knife, showcasing its low density and malleability.
-
Reactivity: Potassium is extremely reactive with water and air. When exposed to air, it rapidly oxidizes, forming a layer of potassium oxide. Reaction with water is even more dramatic, resulting in the production of hydrogen gas and heat, often igniting the hydrogen. This high reactivity necessitates careful handling and storage under inert conditions, often submerged in mineral oil to prevent contact with air and moisture.
-
Melting and Boiling Points: Potassium has a relatively low melting point (63.5 °C) and boiling point (774 °C). These low values are consistent with its position in the periodic table and its metallic bonding characteristics.
-
Electrical Conductivity: Like other metals, potassium is an excellent conductor of electricity. This property is a direct result of the presence of freely moving electrons in its metallic structure. This high conductivity is exploited in various applications.
-
Biological Role: Potassium is an essential element for all living organisms. It plays a vital role in maintaining proper fluid balance, nerve impulse transmission, and muscle contraction. Inadequate potassium levels can lead to various health problems.
Potassium's Role in Biological Systems
Potassium's biological significance cannot be overstated. Its presence is crucial for maintaining the delicate balance of electrolytes within cells and bodily fluids. This balance is vital for numerous physiological processes:
-
Nerve Impulse Transmission: Potassium ions (K⁺) are crucial for the transmission of nerve impulses. The movement of potassium ions across cell membranes generates the electrical signals that allow for communication between nerve cells and the coordination of bodily functions. Disruptions in potassium levels can severely impair nerve function.
-
Muscle Contraction: Similar to nerve impulse transmission, muscle contraction relies heavily on the movement of potassium ions across muscle cell membranes. This ion movement triggers the muscle fiber contraction that enables movement and various other bodily actions. Potassium imbalances can lead to muscle weakness and cramps.
-
Fluid Balance: Potassium plays a crucial role in maintaining the correct balance of fluids within and outside cells. It contributes to the osmotic pressure that regulates the movement of water across cell membranes. Imbalances in potassium levels can disrupt this balance, leading to fluid retention or excessive fluid loss.
-
Enzyme Activation: Potassium acts as a cofactor for certain enzymes, meaning it helps these enzymes function properly. Enzymes are crucial for many metabolic reactions and processes, so proper potassium levels are essential for overall cellular function.
Sources of Potassium and Dietary Considerations
Potassium is widely distributed in nature, found in various foods and minerals. Essential sources for dietary intake include:
-
Fruits and Vegetables: Bananas, avocados, potatoes, tomatoes, and leafy green vegetables are excellent sources of potassium.
-
Legumes: Beans, lentils, and peas are rich in potassium.
-
Dairy Products: Milk and yogurt contain significant amounts of potassium.
-
Meats and Fish: Though generally lower in potassium compared to plant-based sources, meats and fish still contribute to overall intake.
Maintaining adequate potassium levels through a balanced diet is vital for optimal health. However, excessive potassium intake can also be problematic, particularly for individuals with kidney problems. It's always advisable to consult with a healthcare professional for personalized dietary advice regarding potassium intake.
Potassium in Industry and Technology
Beyond its biological importance, potassium also holds several industrial and technological applications. Its reactivity and properties make it useful in:
-
Fertilizers: Potassium compounds are essential components of fertilizers, providing the potassium needed for healthy plant growth.
-
Glass Production: Potassium compounds are used in the production of specialized types of glass.
-
Soap and Detergents: Potassium hydroxide is employed in the manufacturing of soap and detergents.
-
Photography: Potassium compounds are utilized in certain photographic processes.
-
Alloying: Potassium is sometimes used in the creation of certain alloys, although its high reactivity often limits its direct application.
Potassium Isotopes and Radioactive Applications
Potassium exists in nature as several isotopes, which are atoms of the same element with different numbers of neutrons. The most common isotopes are ³⁹K (93.3%), ⁴⁰K (0.012%), and ⁴¹K (6.7%). Notably, ⁴⁰K is a radioactive isotope that undergoes radioactive decay. This decay is utilized in various applications:
-
Geological Dating: The decay rate of ⁴⁰K is used in radiometric dating to determine the age of rocks and minerals. This method provides valuable insights into geological processes and the Earth's history.
-
Medical Imaging: In limited circumstances, radioactive potassium isotopes are used in certain medical imaging techniques. However, the use of radioactive isotopes in medical applications needs strict safety protocols.
Conclusion: The Significance of Understanding Potassium
The atomic symbol for potassium, K, represents much more than just a simple abbreviation. It symbolizes the rich history of scientific discovery, the significance of precise chemical notation, and the essential role of this element in both biological systems and industrial applications. From its dramatic reactivity to its crucial involvement in life processes, potassium continues to fascinate and intrigue scientists and researchers worldwide. A thorough understanding of its properties, uses, and biological significance provides valuable insights into chemistry, biology, geology, and various other scientific disciplines. The detailed exploration of potassium's atomic symbol, therefore, serves as a gateway to a deeper appreciation of this essential element and its impact on our world.
Latest Posts
Latest Posts
-
What Happens To Mrna After It Completes Transcription
Mar 26, 2025
-
What Is The Lcm Of 2 And 3
Mar 26, 2025
-
A Group Of Fish Is Called
Mar 26, 2025
-
Which One Of The Following Statements Is False
Mar 26, 2025
-
Which Of Following Statements Is True
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Atomic Symbol For Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
