What Is Steric Hindrance In Organic Chemistry
Juapaving
Mar 28, 2025 · 6 min read
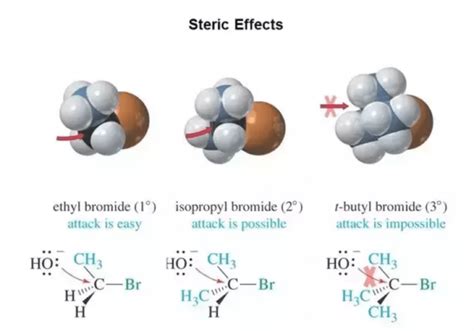
Table of Contents
What is Steric Hindrance in Organic Chemistry? A Comprehensive Guide
Steric hindrance, a cornerstone concept in organic chemistry, significantly influences the reactivity and properties of molecules. Understanding steric hindrance is crucial for predicting reaction outcomes, designing efficient synthetic routes, and interpreting the behavior of complex organic systems. This comprehensive guide delves into the intricacies of steric hindrance, exploring its definition, causes, consequences, and applications across various organic chemistry domains.
Defining Steric Hindrance: Space Matters in Chemical Reactions
At its core, steric hindrance refers to the impediment of a chemical reaction due to the physical bulkiness of substituent groups attached to a reacting atom or molecule. These bulky groups physically block or hinder the approach of other reactants, slowing down or even preventing the reaction from occurring. Imagine trying to fit a large sofa through a narrow doorway – the sofa's size (steric bulk) hinders its passage. Similarly, bulky substituents hinder the approach of reagents to the reactive center of a molecule.
The Role of Molecular Shape and Size
Steric hindrance isn't simply about the size of substituents; their shape and orientation also play crucial roles. A linear alkyl group might cause less steric hindrance than a branched alkyl group of comparable size, due to differences in their spatial arrangement. The three-dimensional structure of the molecule, often visualized using models like ball-and-stick or space-filling models, is essential for understanding steric effects.
Causes of Steric Hindrance: Bulky Groups and Crowded Environments
Several factors contribute to the manifestation of steric hindrance:
1. Bulky Substituents: The Bigger, the Better (at Hindering)
The most obvious cause is the presence of large substituent groups attached to the reactive center. Examples include tert-butyl groups, phenyl groups, and other large alkyl chains. These groups occupy significant space, physically obstructing the approach of reactants.
2. Conformation and Configuration: The Impact of 3D Structure
The spatial arrangement of atoms in a molecule (its conformation and configuration) greatly influences the degree of steric hindrance. Certain conformations may bring bulky groups closer together, exacerbating steric interactions, while others may alleviate the hindrance. For example, cis- and trans- isomers of alkenes often exhibit different reactivities due to variations in steric hindrance.
3. Ring Strain: Crowding in Cyclic Systems
Cyclic molecules, especially those with small rings (e.g., three- or four-membered rings), often experience significant steric strain. The bond angles are compressed, leading to repulsive interactions between substituents and a greater tendency for steric hindrance.
4. Proximity of Reactive Centers: Close Encounters of the Steric Kind
When multiple reactive centers are located close to each other in a molecule, steric hindrance can become pronounced. The approach of a reactant to one center may be blocked by the proximity of another, resulting in reduced reactivity.
Consequences of Steric Hindrance: Impact on Reactivity and Properties
Steric hindrance exerts a profound influence on various aspects of a molecule's behavior:
1. Reduced Reaction Rates: Slower Reactions
The most immediate consequence is a decrease in the rate of chemical reactions. The steric bulk hinders the formation of the transition state, the high-energy intermediate formed during the reaction, thereby increasing the activation energy and slowing down the reaction.
2. Altered Reaction Pathways: Reactions Take Different Routes
Steric hindrance can sometimes force a reaction to proceed via a different mechanistic pathway compared to an unhindered reaction. This may lead to the formation of unexpected products or the suppression of certain reaction types.
3. Changes in Product Selectivity: Favoring Certain Products
In reactions yielding multiple products, steric hindrance can influence the selectivity of the reaction, favoring the formation of one product over another. Bulky groups can preferentially shield certain reactive sites, directing the reaction towards a specific product.
4. Modifications of Physical Properties: Melting Points, Boiling Points, and More
Steric hindrance can also impact the physical properties of molecules. For example, increased steric bulk may lead to higher melting points and boiling points due to reduced intermolecular interactions. It can also influence solubility and other physical characteristics.
Examples of Steric Hindrance in Action: Real-World Applications
Steric hindrance is a ubiquitous phenomenon in organic chemistry, impacting a vast array of reactions and molecules. Here are a few illustrative examples:
1. SN1 vs. SN2 Reactions: A Tale of Two Mechanisms
Steric hindrance plays a crucial role in determining whether a nucleophilic substitution reaction follows an SN1 (unimolecular) or SN2 (bimolecular) mechanism. SN2 reactions, which involve a backside attack of the nucleophile, are significantly inhibited by steric hindrance at the reaction center. Therefore, bulky substrates tend to favor the SN1 mechanism, which involves a carbocation intermediate.
2. The Electrophilic Aromatic Substitution: Directing Effects of Substituents
In electrophilic aromatic substitution reactions, the steric bulk of substituents on the aromatic ring influences the regioselectivity of the reaction. Bulky groups can hinder the approach of the electrophile to certain positions on the ring, favoring substitution at less hindered sites.
3. Grignard Reactions: Steric Effects on Reactivity
Grignard reagents, organomagnesium compounds, are highly reactive nucleophiles. However, steric hindrance can significantly reduce their reactivity, making it difficult to perform Grignard reactions on highly hindered substrates.
4. Polymer Chemistry: Controlling Polymerization via Steric Hindrance
Steric hindrance plays a crucial role in polymer chemistry, influencing the rate of polymerization and the properties of the resulting polymers. Bulky substituents can inhibit chain growth, leading to polymers with lower molecular weights and altered physical properties.
5. Enzyme Catalysis: Shape Selectivity and Active Sites
In biological systems, steric hindrance plays a vital role in enzyme catalysis. The active site of an enzyme is often a pocket with specific steric constraints, ensuring that only the correct substrate can bind and undergo catalysis. This exquisite shape selectivity is crucial for the specificity and efficiency of enzyme-catalyzed reactions.
Overcoming Steric Hindrance: Strategies and Techniques
While steric hindrance can be a significant obstacle in organic synthesis, several strategies can be employed to overcome it:
1. Choosing Appropriate Reagents and Reaction Conditions: Optimizing the Environment
Using smaller, less bulky reagents or altering reaction conditions (e.g., temperature, solvent) can sometimes alleviate steric hindrance.
2. Protecting Groups: Temporarily Shielding Reactive Sites
Protecting groups can be used to temporarily block reactive sites, allowing other parts of the molecule to undergo reactions without interference from steric hindrance.
3. Catalyst Design: Enhancing Reactivity Through Catalysis
Enzymes and other catalysts can overcome steric hindrance by binding to substrates in a specific orientation that reduces steric interactions. Designing effective catalysts for sterically hindered reactions remains an active area of research.
4. Computational Chemistry: Predicting Steric Effects and Optimizing Synthesis
Computational methods can be used to predict steric hindrance and guide the design of efficient synthetic routes, minimizing steric interactions and enhancing reaction yields.
Conclusion: The Significance of Steric Hindrance in Organic Chemistry
Steric hindrance is an indispensable concept in organic chemistry, profoundly influencing reactivity, selectivity, and the physical properties of molecules. Understanding the principles of steric hindrance is crucial for designing successful synthetic strategies, interpreting experimental results, and predicting the behavior of complex organic systems. From seemingly simple reactions to intricate biological processes, the influence of steric effects permeates all aspects of the field, making its mastery essential for any aspiring organic chemist. Continued research into understanding and manipulating steric effects will undoubtedly continue to shape the future of organic chemistry, leading to the development of novel synthetic methods and the design of new materials with tailored properties.
Latest Posts
Latest Posts
-
How Many Feet Is 45 Inches
Mar 31, 2025
-
Is The Square Root Of 12 Rational Or Irrational
Mar 31, 2025
-
What Is The Prime Factor Of 135
Mar 31, 2025
-
The Image Seen In A Plane Mirror Is Located
Mar 31, 2025
-
How Are Combustion And Cellular Respiration Different
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is Steric Hindrance In Organic Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
