Acids Turn Litmus Paper What Color
Juapaving
Mar 24, 2025 · 7 min read
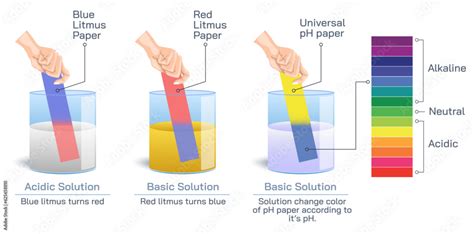
Table of Contents
Acids Turn Litmus Paper What Color? Understanding pH Indicators
The question, "Acids turn litmus paper what color?" is a fundamental one in chemistry, touching upon the concept of pH and the use of indicators to determine the acidity or alkalinity of a solution. The simple answer is red. However, understanding why this happens requires a deeper dive into the chemistry behind litmus paper and its interaction with acids and bases. This comprehensive guide will explore the topic thoroughly, covering the nature of acids and bases, the mechanism of litmus paper, its applications, and related concepts.
What is Litmus Paper?
Litmus paper is a crucial tool in chemistry and related fields for quickly determining whether a substance is acidic or basic (alkaline). It's a type of pH indicator, meaning it changes color depending on the pH of the solution it's dipped in. The paper is made from a mixture of different dyes extracted from lichens, a type of fungus. These dyes change color based on the hydrogen ion (H⁺) concentration in a solution. A low hydrogen ion concentration indicates a basic solution, while a high concentration points to an acidic one.
The key to litmus paper's functionality lies in its ability to interact with hydrogen ions. The dye molecules in the paper have different chemical structures that exist in different forms depending on the solution's pH. These forms have different colors, allowing for visual identification of the solution's acidity or alkalinity.
Acids and Bases: A Quick Refresher
Before delving deeper into the color change mechanism, let's briefly revisit the concepts of acids and bases.
Defining Acids
Acids are substances that donate protons (H⁺ ions) when dissolved in water. They typically have a sour taste, react with metals to produce hydrogen gas, and turn blue litmus paper red. Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), completely dissociate in water, releasing a high concentration of H⁺ ions. Weak acids, like acetic acid (CH₃COOH), partially dissociate, releasing fewer H⁺ ions.
Defining Bases
Bases are substances that accept protons (H⁺ ions) or donate hydroxide ions (OH⁻ ions) when dissolved in water. They typically have a bitter taste, feel slippery, and turn red litmus paper blue. Strong bases, like sodium hydroxide (NaOH) and potassium hydroxide (KOH), completely dissociate in water, releasing a high concentration of OH⁻ ions. Weak bases, like ammonia (NH₃), partially dissociate, releasing fewer OH⁻ ions.
The Chemistry of the Color Change: How Acids Turn Litmus Paper Red
The color change of litmus paper is a result of a reversible chemical reaction between the dye molecules and the hydrogen ions (H⁺) in the solution. When litmus paper is dipped in an acidic solution, the high concentration of H⁺ ions interacts with the dye molecules. This interaction causes a change in the dye's molecular structure, resulting in a shift in the wavelengths of light it absorbs and reflects. This shift manifests as a change in color.
The specific dyes used in litmus paper are complex organic molecules with several chemical groups that can donate or accept electrons. The presence of H⁺ ions affects the electron distribution within these molecules. The acidic environment leads to a protonation of specific groups within the dye molecules. This protonation alters the electronic structure of the dye, which ultimately leads to the color change from blue to red. This change is visually observable and indicates the acidic nature of the solution.
Why Red for Acids? The Role of the Dye Molecules
The exact mechanism of the color change depends on the precise composition of the dye mixture within the litmus paper. However, the general principle involves the structural transformation of the dye molecules in response to the surrounding pH. Different dyes respond differently to changes in pH, resulting in a range of color changes. The particular dyes selected for litmus paper exhibit a distinct color shift to red under acidic conditions. This is a specific property of the dyes used and is not a universal characteristic of all pH indicators.
Litmus Paper: More Than Just a Red/Blue Indicator
While the red color change in response to acids is the most commonly known aspect of litmus paper, it's important to note that it's not solely a binary indicator of acid/base. It provides a broad indication of pH range. Though it doesn’t provide a precise pH value, the color change itself can be used to differentiate solutions into acidic or basic categories.
Beyond Red and Blue: Shades of Color
The color change isn't simply a sharp transition from blue to red. It often involves various shades of purple, indicating a transitional pH zone. This transitional phase is where the solution's pH is near neutral (approximately pH 7). In this range, the litmus paper might appear purple or a combination of red and blue, reflecting the coexistence of different forms of the dye molecules. It shows that the litmus test offers a visual representation of a pH gradient rather than just a simple acid or base identification.
Applications of Litmus Paper
Litmus paper finds wide applications across various fields:
-
Chemistry Education: It serves as an essential tool in educational settings for demonstrating basic concepts of acids and bases. It's often used in schools and colleges to teach students how to identify and differentiate between acidic and basic solutions.
-
Environmental Monitoring: Litmus paper can provide quick assessments of soil and water acidity, which is crucial in agriculture and environmental studies. Testing soil pH helps farmers select suitable crops and manage soil health effectively. Similarly, monitoring water pH aids in determining water quality and preventing environmental damage.
-
Industrial Processes: Many industrial processes require specific pH levels for optimal performance. Litmus paper can offer a rapid, albeit less precise, method of monitoring pH in real-time, allowing for timely adjustments as needed.
-
Food and Beverage Industry: pH control is crucial in food and beverage production. Litmus paper can provide a basic pH check during various stages of production to ensure product quality and safety.
-
Medical Applications: Although not a primary tool, litmus paper can be used for preliminary pH checks in some medical contexts, such as evaluating body fluids. However, more precise methods are usually employed for accurate medical diagnostics.
Limitations of Litmus Paper
Despite its usefulness, litmus paper does have limitations:
-
Imprecise pH Measurement: Litmus paper only provides a qualitative assessment of pH, indicating whether a substance is acidic or basic, without providing a precise pH value. For accurate pH determination, a pH meter is needed.
-
Limited pH Range: The color change of litmus paper is only reliable within a specific pH range. Outside this range, the color change may be ambiguous or unreliable.
-
Interference from Other Substances: Certain substances can interfere with the color change of litmus paper, leading to inaccurate results. The presence of other color-changing compounds in the sample can mask the true color change of the litmus paper itself.
Alternatives to Litmus Paper: Other pH Indicators
Besides litmus paper, various other pH indicators are used for determining acidity or alkalinity. These include:
-
Universal indicator: This is a mixture of several indicators that provide a broader color range, allowing for a more approximate pH value estimation.
-
Methyl orange: This indicator changes color from red in acidic solutions to yellow in basic solutions.
-
Phenolphthalein: This indicator is colorless in acidic solutions and turns pink in basic solutions.
-
Bromothymol blue: This indicator changes color from yellow in acidic solutions to blue in basic solutions, passing through a green shade near neutral pH.
Each of these indicators has its specific color change range and sensitivity, making them suitable for different applications.
Conclusion: Litmus Paper – A Simple Yet Powerful Tool
The answer to "Acids turn litmus paper what color?" is unequivocally red. However, the underlying chemistry and the broader implications of this color change are much richer. Litmus paper, a simple yet effective tool, provides a quick and accessible method for differentiating between acidic and basic solutions. While it has limitations in terms of precision and range, it continues to hold significant value in education, environmental monitoring, and various industrial applications. Understanding its limitations and exploring alternative indicators provides a more complete picture of pH measurement and the versatile applications of different pH indicators in various scientific and industrial settings.
Latest Posts
Latest Posts
-
John Drives To His Workplace And Back Home
Mar 25, 2025
-
What Is The Division Of Cytoplasm Called
Mar 25, 2025
-
3 1 8 As A Decimal
Mar 25, 2025
-
What Is A Factor Of 5
Mar 25, 2025
-
List Three Similarities Between Dna And Rna
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Acids Turn Litmus Paper What Color . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
