What Is Oxidized And Reduced In Cellular Respiration
Juapaving
Mar 09, 2025 · 7 min read
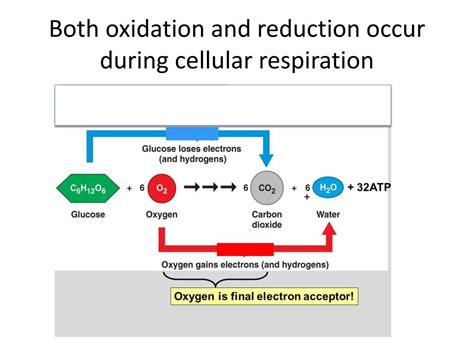
Table of Contents
What is Oxidized and Reduced in Cellular Respiration?
Cellular respiration is a fundamental process in all living organisms, responsible for converting the chemical energy stored in glucose into a readily usable form of energy called ATP (adenosine triphosphate). This intricate process involves a series of redox reactions, where electrons are transferred from one molecule to another. Understanding what is oxidized and reduced during cellular respiration is crucial to grasping the entire mechanism.
The Fundamentals of Redox Reactions
Before delving into the specifics of cellular respiration, let's establish a clear understanding of oxidation and reduction. These terms, often abbreviated as redox reactions, describe the transfer of electrons between atoms or molecules.
-
Oxidation: This involves the loss of electrons by a molecule or atom. A molecule that loses electrons is said to be oxidized. Often, oxidation is accompanied by an increase in oxidation state (a measure of the degree of oxidation of an atom in a molecule).
-
Reduction: This involves the gain of electrons by a molecule or atom. A molecule that gains electrons is said to be reduced. Reduction is accompanied by a decrease in oxidation state.
Remember the mnemonic: OIL RIG – Oxidation Is Loss, Reduction Is Gain (of electrons).
Stages of Cellular Respiration and Redox Reactions
Cellular respiration is broadly divided into four main stages:
- Glycolysis: The breakdown of glucose in the cytoplasm.
- Pyruvate Oxidation: Conversion of pyruvate to acetyl-CoA in the mitochondrial matrix.
- Krebs Cycle (Citric Acid Cycle): A cyclical series of redox reactions in the mitochondrial matrix.
- Electron Transport Chain (ETC) and Oxidative Phosphorylation: Electron transport and ATP synthesis across the inner mitochondrial membrane.
Let's examine each stage and pinpoint the molecules undergoing oxidation and reduction:
1. Glycolysis: Oxidation of Glucose, Reduction of NAD+
Glycolysis takes place in the cytoplasm and doesn't require oxygen. It involves a series of enzyme-catalyzed reactions that break down a six-carbon glucose molecule into two three-carbon pyruvate molecules. Crucially, this process involves redox reactions:
-
Glucose Oxidation: Glucose is oxidized. Specifically, some of its carbon atoms lose electrons and become more oxidized. This is not a direct electron transfer to another molecule in a single step, but rather a series of coupled reactions where electrons are passed onto coenzymes.
-
NAD+ Reduction: Nicotinamide adenine dinucleotide (NAD+), an important electron carrier, is reduced to NADH. NAD+ accepts two electrons and a proton (H+), becoming NADH. This NADH carries the electrons obtained from the oxidation of glucose to the next stage of cellular respiration.
In essence: Glucose is oxidized, and NAD+ is reduced during glycolysis. The energy released during glucose oxidation is partially captured in the high-energy electrons carried by NADH.
2. Pyruvate Oxidation: Further Oxidation, More NADH Production
Pyruvate, the product of glycolysis, is transported into the mitochondrial matrix. Here, it undergoes pyruvate oxidation, a transitional step before entering the Krebs cycle.
-
Pyruvate Oxidation: Pyruvate is further oxidized. It loses a carbon atom as carbon dioxide (CO2), a byproduct of cellular respiration. The remaining two-carbon fragment is combined with coenzyme A (CoA) to form acetyl-CoA.
-
NAD+ Reduction: Again, NAD+ is reduced to NADH during this oxidation process. The electrons released from the oxidation of pyruvate are transferred to NAD+.
3. Krebs Cycle (Citric Acid Cycle): A Cycle of Oxidations and Reductions
The Krebs cycle is a central metabolic pathway in the mitochondrial matrix. It is a cyclical series of reactions where acetyl-CoA is completely oxidized to CO2.
The cycle involves a series of redox reactions where different molecules are oxidized and reduced:
-
Acetyl-CoA Oxidation: Acetyl-CoA is fully oxidized to CO2. Its carbon atoms lose electrons as they are converted to CO2.
-
NAD+ and FAD Reduction: Both NAD+ and flavin adenine dinucleotide (FAD), another electron carrier, are reduced. NAD+ is reduced to NADH, and FAD is reduced to FADH2. These electron carriers transport electrons from the Krebs cycle to the electron transport chain.
-
Oxidation of Intermediates: Several intermediate molecules within the cycle undergo oxidation and reduction.
4. Electron Transport Chain (ETC) and Oxidative Phosphorylation: The Final Electron Transfer
The electron transport chain (ETC) is a series of protein complexes embedded in the inner mitochondrial membrane. The electrons carried by NADH and FADH2 from the previous stages are passed along the ETC.
-
Electron Transfer and Proton Pumping: As electrons move down the ETC, energy is released. This energy is used to pump protons (H+) from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space, creating a proton gradient.
-
Oxygen as the Final Electron Acceptor: Oxygen (O2) acts as the terminal electron acceptor at the end of the ETC. It accepts electrons and combines with protons to form water (H2O), a byproduct of cellular respiration. This is why oxygen is essential for aerobic respiration.
-
Oxidative Phosphorylation: The proton gradient generated by the ETC drives ATP synthesis through chemiosmosis. Protons flow back into the matrix through ATP synthase, an enzyme that uses the energy of this proton flow to produce ATP. This process is called oxidative phosphorylation. Although not strictly a redox reaction, it directly relies on the redox reactions of the ETC.
In Summary: In the ETC, NADH and FADH2 are oxidized (lose electrons), while oxygen is reduced (gains electrons) to form water. The energy released during electron transport fuels ATP synthesis.
Overall Redox Balance in Cellular Respiration
The entire process of cellular respiration involves a complex interplay of oxidation and reduction reactions. Glucose is fully oxidized to CO2, releasing energy. This energy is used to reduce NAD+ and FAD to NADH and FADH2, respectively. The electrons carried by these electron carriers are then used to reduce oxygen to water in the ETC, generating ATP through oxidative phosphorylation.
The net equation for cellular respiration summarizes this:
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
This equation shows that glucose (C6H12O6) is oxidized, and oxygen (O2) is reduced. The carbon atoms in glucose are oxidized to carbon dioxide, while oxygen gains electrons to form water. The energy released from this redox process is stored in the form of ATP, the cell's energy currency.
Significance of Redox Reactions in Cellular Respiration
Redox reactions are fundamental to cellular respiration because they allow for the controlled release of energy from glucose. The step-wise transfer of electrons prevents the sudden release of large amounts of energy as heat, which would be inefficient and potentially damaging to the cell. Instead, the energy is released gradually and harnessed to generate ATP, the usable form of energy for cellular processes.
The involvement of electron carriers like NAD+ and FAD is also vital. These molecules act as intermediates, accepting and donating electrons in a controlled manner, facilitating the efficient transfer of energy throughout the process. The final acceptance of electrons by oxygen is essential for the continuous operation of the ETC and ATP synthesis.
Understanding Oxidation and Reduction: A Deeper Dive
It's crucial to note that oxidation and reduction aren't always about the direct transfer of electrons. In some cases, oxidation can be defined as the increase in oxidation state, which often involves the loss of hydrogen atoms or the gain of oxygen atoms. Conversely, reduction can be defined as the decrease in oxidation state, which often involves the gain of hydrogen atoms or the loss of oxygen atoms. These alternative definitions are helpful in situations where electron transfers are not directly observable.
Conclusion: The Elegant Dance of Electrons in Life's Engine
Cellular respiration is a magnificent example of the power of redox reactions in biological systems. The carefully orchestrated oxidation and reduction steps ensure efficient energy extraction from glucose, powering all life processes. Understanding the specific molecules that are oxidized and reduced during each stage allows for a comprehensive understanding of how this fundamental process sustains life. The intricate balance and transfer of electrons make cellular respiration a truly remarkable and elegantly efficient system. Further exploration into the specifics of the enzymes and mechanisms involved only deepens appreciation for the complexity and sophistication of this essential biological process.
Latest Posts
Latest Posts
-
According To The Law Of Conservation Of Mass
Mar 09, 2025
-
What Are The Factors For 13
Mar 09, 2025
-
How To Get The Diameter Of A Sphere
Mar 09, 2025
-
What Color Is An Animal Cell
Mar 09, 2025
-
What Is The Lcm For 5 And 9
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Is Oxidized And Reduced In Cellular Respiration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
