What Is One Difference Between A Mixture And A Compound
Juapaving
Mar 28, 2025 · 6 min read
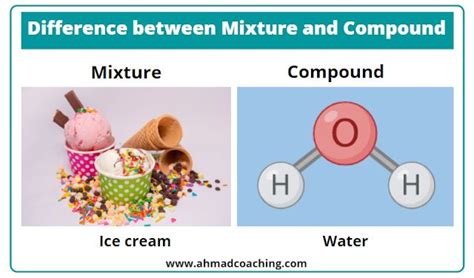
Table of Contents
What is One Key Difference Between a Mixture and a Compound?
The seemingly simple question of differentiating between mixtures and compounds often trips up students and even seasoned science enthusiasts. While both are combinations of substances, the fundamental difference lies in how those substances are combined. Understanding this core distinction is crucial for grasping a wide range of scientific concepts, from basic chemistry to advanced materials science. This article will delve deep into this crucial difference, exploring the properties, formation, and examples of both mixtures and compounds to provide a comprehensive understanding.
The Defining Difference: Chemical vs. Physical Combination
The single most significant difference between a mixture and a compound lies in the type of combination involved:
-
Mixtures are formed by physically combining different substances. The individual components retain their original chemical identities and properties. No new substance is created. Think of it like mixing sand and sugar – you can still see and separate the sand and sugar particles.
-
Compounds, on the other hand, are formed by chemically combining different elements. This involves a chemical reaction where the original elements lose their individual properties and form a new substance with entirely different characteristics. The components are bonded together at the atomic or molecular level, creating a distinct chemical entity. For example, when hydrogen and oxygen react to form water (H₂O), the properties of hydrogen and oxygen are completely lost in the formation of the new compound, water.
Mixtures: A Closer Look
Mixtures are ubiquitous in our daily lives. Everything from the air we breathe to the food we eat is essentially a mixture. There are two main types of mixtures:
1. Homogenous Mixtures: Uniformity at a Microscopic Level
Homogenous mixtures appear uniform throughout. At a microscopic level, the components are evenly distributed, and you cannot visually distinguish between them. Examples include:
- Air: A mixture of gases like nitrogen, oxygen, argon, and carbon dioxide.
- Saltwater: Dissolved salt is uniformly dispersed throughout the water.
- Brass: An alloy of copper and zinc, appearing as a single, solid phase.
- Sugar dissolved in water: The sugar molecules are evenly distributed within the water molecules.
2. Heterogeneous Mixtures: Visible Differences
In contrast to homogeneous mixtures, heterogeneous mixtures display visible differences in their composition. Different components are easily distinguishable and may exist in separate phases (solid, liquid, or gas). Examples include:
- Sand and water: The sand particles are clearly visible and settle to the bottom of the container.
- Oil and water: Oil and water do not mix and form distinct layers.
- Granite: A rock containing visibly different minerals like quartz, feldspar, and mica.
- A salad: Clearly distinguishable components of vegetables, fruits, and dressing.
Compounds: The Realm of Chemical Bonds
Compounds, unlike mixtures, involve the formation of chemical bonds between atoms. These bonds hold the atoms together to form molecules or crystals with unique properties. The properties of a compound are entirely different from the properties of its constituent elements. Consider the following:
- Water (H₂O): Hydrogen is a highly flammable gas, and oxygen is a crucial component for combustion. Yet, their combination forms water, a substance that extinguishes fire.
- Sodium Chloride (NaCl): Sodium is a highly reactive metal, and chlorine is a poisonous gas. Their reaction forms sodium chloride (table salt), a staple in our diet.
- Carbon Dioxide (CO₂): Carbon is a solid element, and oxygen is a gas. Together they form carbon dioxide, a crucial greenhouse gas.
- Ammonia (NH₃): Nitrogen is a relatively inert gas, and hydrogen is highly flammable. Ammonia is a pungent-smelling gas used in many industrial applications.
The formation of chemical bonds in compounds results in fixed ratios of elements. For instance, water always has a 2:1 ratio of hydrogen to oxygen atoms (H₂O). This fixed ratio is a defining characteristic that distinguishes compounds from mixtures.
Separating Mixtures vs. Separating Compounds
Another key difference lies in the methods required for separation. Mixtures can be separated using physical methods that don't alter the chemical composition of the components. These methods include:
- Filtration: Separating solids from liquids (e.g., separating sand from water).
- Distillation: Separating liquids based on their boiling points (e.g., separating water from saltwater).
- Evaporation: Separating a dissolved solid from a liquid (e.g., obtaining salt from saltwater).
- Chromatography: Separating components based on their different affinities for a stationary and mobile phase.
- Decantation: Pouring off a liquid from a sediment.
- Magnetism: Separating magnetic materials from non-magnetic ones.
Separating compounds, however, requires chemical methods, such as chemical reactions or electrolysis. These methods break the chemical bonds holding the compound together, resulting in the formation of new substances. For example, the electrolysis of water uses an electric current to break water molecules into hydrogen and oxygen gases.
Properties of Mixtures vs. Compounds
The properties of mixtures and compounds also differ significantly.
Mixtures:
- Variable composition: The ratio of components can vary widely.
- Retain individual properties: Components retain their original characteristics.
- Easily separable: Separable using physical methods.
- No energy change upon mixing: Minimal or no heat is released or absorbed during mixing.
Compounds:
- Fixed composition: A definite and constant ratio of elements.
- New properties: Properties different from the constituent elements.
- Difficult to separate: Separable only by chemical methods.
- Energy change during formation: Heat is often released or absorbed during the formation of a compound.
Examples in Everyday Life: Mixtures and Compounds Around Us
Let's examine some everyday examples to solidify our understanding:
Mixtures:
- Coffee: A mixture of coffee grounds, water, and potentially milk and sugar.
- Soil: A mixture of sand, silt, clay, organic matter, and minerals.
- Paint: A mixture of pigments, binders, and solvents.
- Blood: A mixture of various cells and plasma.
- Milk: A mixture of water, fat, proteins, and carbohydrates.
Compounds:
- Water (H₂O): Essential for life, with vastly different properties than hydrogen and oxygen.
- Table salt (NaCl): A crucial nutrient, unlike its constituent elements, sodium and chlorine.
- Sugar (C₁₂H₂₂O₁₁): A source of energy, different from carbon, hydrogen, and oxygen individually.
- Carbon dioxide (CO₂): A greenhouse gas, different from carbon and oxygen.
- Baking soda (NaHCO₃): Used in baking, vastly different from its individual components.
Conclusion: Understanding the Fundamental Difference
The core distinction between a mixture and a compound lies in the nature of the combination – physical versus chemical. Mixtures involve a physical combination where individual components retain their identities, while compounds involve a chemical reaction forming a new substance with unique properties. Understanding this fundamental difference is essential for navigating the world of chemistry and appreciating the intricate relationships between matter and its various forms. This difference underlies countless chemical processes and explains the diverse properties of substances around us, highlighting the crucial role of chemical bonding in shaping the material world. By mastering the concepts presented here, one can approach more complex scientific concepts with greater ease and confidence.
Latest Posts
Latest Posts
-
How Many Bonds Does Sulfur Have
Mar 31, 2025
-
Prime Numbers Between 80 And 90
Mar 31, 2025
-
What Is The Prime Factorization Of 420
Mar 31, 2025
-
Hansel And Gretel Short Story Pdf
Mar 31, 2025
-
Is There Friction In Outer Space
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is One Difference Between A Mixture And A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
