How Many Bonds Does Sulfur Have
Juapaving
Mar 31, 2025 · 5 min read
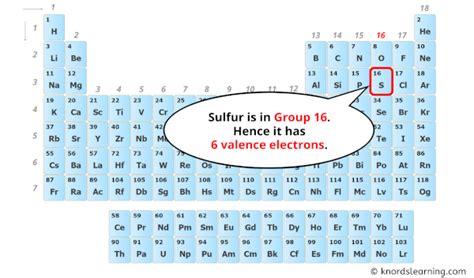
Table of Contents
How Many Bonds Does Sulfur Have? Exploring the Bonding Versatility of a Key Element
Sulfur, a vibrant yellow nonmetal abundant in nature, plays a crucial role in various biological and industrial processes. Its unique electronic structure allows for a remarkable versatility in bonding, leading to a wide range of compounds and structures. Understanding sulfur's bonding capabilities is key to comprehending its diverse chemical behavior. This article delves into the intricacies of sulfur bonding, exploring the factors influencing its bond formation and highlighting examples across various chemical contexts.
Sulfur's Electronic Configuration: The Foundation of Bonding
The answer to "how many bonds does sulfur have?" isn't a simple numerical response. It's contingent on several factors, primarily its electronic configuration. Sulfur (S), with an atomic number of 16, boasts an electronic configuration of 1s²2s²2p⁶3s²3p⁴. This configuration reveals that sulfur has six valence electrons – electrons in its outermost shell actively participating in chemical bonding.
These six valence electrons are crucial in determining sulfur's bonding capacity. While it can seemingly form up to six bonds, the reality is far more nuanced. The stability of the resulting molecule and the electronegativity of other atoms involved heavily influence the number of bonds sulfur ultimately forms.
The Octet Rule and Sulfur's Exceptions
The octet rule, a fundamental principle in chemistry, suggests that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their valence shell. While this rule serves as a useful guideline, sulfur frequently defies it. Because of its position in the periodic table and the availability of its 3d orbitals, sulfur can expand its octet, accommodating more than eight electrons in its valence shell. This capability allows for sulfur to exhibit hypervalency, forming more than four bonds.
Sulfur's Common Bonding Numbers: A Detailed Look
Although sulfur can exceed the octet rule, certain bonding numbers are far more prevalent than others. Let's examine them:
Two Bonds: Sulfides and Disulfides
Sulfur commonly forms two bonds, leading to a variety of compounds. A classic example lies in sulfides, where sulfur shares electrons with two other atoms, achieving a stable configuration. Think of hydrogen sulfide (H₂S), a foul-smelling gas found in natural gas and volcanic emissions. Here, sulfur forms two single bonds, one with each hydrogen atom.
Another crucial example illustrating two-bond sulfur is the formation of disulfide bonds. These covalent bonds play a critical role in protein structure, where two cysteine residues (containing thiol groups, -SH) link together, forming a disulfide bridge (-S-S-). These disulfide bridges significantly impact protein folding and stability.
Four Bonds: Sulfates and Sulfonates
Sulfur's ability to form four bonds is also widely observed. This often involves double bonds to oxygen atoms. A quintessential example lies in the sulfate ion (SO₄²⁻), a crucial anion in various chemical and biological contexts. In this case, sulfur is at the center, forming two double bonds and two single bonds to oxygen atoms, completing its octet.
Similarly, sulfonates (R-SO₃⁻) are a class of compounds where sulfur forms four bonds – one to a carbon atom (R) and three to oxygen atoms (with one oxygen atom double-bonded). Sulfonates find numerous applications, including detergents and surfactants.
Six Bonds: Hypervalent Sulfur Compounds
Moving beyond the octet rule, sulfur frequently exhibits hypervalency, forming six bonds. These compounds are often characterized by sulfur's involvement in multiple bonds with electronegative atoms, typically oxygen.
Sulfuric acid (H₂SO₄) is a prime example of a hypervalent sulfur compound. Here, sulfur forms two double bonds and two single bonds with oxygen atoms, resulting in a molecule featuring two hydroxyl groups (-OH) and two double-bonded oxygens. This makes sulfuric acid a powerful oxidizing agent and a cornerstone chemical in numerous industrial processes.
Other examples of six-bond sulfur compounds include sulfur hexafluoride (SF₆) and various other sulfur-fluorine compounds. The high electronegativity of fluorine helps stabilize the expanded octet around sulfur.
Factors Influencing Sulfur's Bonding Behavior
Several factors influence the number of bonds sulfur forms:
-
Electronegativity of the bonding partner: More electronegative atoms, like oxygen and fluorine, can more effectively attract electrons, enabling sulfur to form more bonds.
-
Steric effects: The size and arrangement of other atoms surrounding sulfur can influence the steric hindrance, affecting the possibility of forming more bonds.
-
Resonance: In many cases, the actual bonding structure in sulfur-containing molecules is a resonance hybrid, a blend of multiple contributing structures. This can stabilize the molecule, even with sulfur forming more than four bonds.
-
Orbital availability: The presence of available 3d orbitals in sulfur allows for the expansion of its valence shell and the formation of hypervalent compounds.
Applications Highlighting Sulfur's Diverse Bonding
The versatile bonding nature of sulfur is evident across diverse applications:
-
Industrial processes: Sulfuric acid, a hypervalent sulfur compound, is a cornerstone chemical in various industrial processes, from fertilizer production to metal refining.
-
Biochemistry: Sulfur plays a vital role in biological systems, forming disulfide bonds that maintain protein structure and function. It's also present in essential amino acids like cysteine and methionine.
-
Pharmaceuticals: Numerous pharmaceuticals incorporate sulfur, leveraging its unique bonding properties and reactivity.
-
Materials science: Sulfur-containing compounds find applications in various materials, including polymers and rubbers.
Conclusion: The Complexity of Sulfur Bonding
The question "how many bonds does sulfur have?" reveals itself to be far more intricate than initially perceived. While the number of bonds sulfur forms varies, it usually ranges from two to six. This versatility stems from its electronic configuration, the influence of surrounding atoms, and its ability to exceed the octet rule. Understanding this complex bonding behavior is crucial for comprehending sulfur's vital role in numerous natural and industrial settings, highlighting its significant importance in the world around us. Further research into sulfur's bonding characteristics continues to unlock new possibilities and deepen our understanding of this essential element.
Latest Posts
Latest Posts
-
How Do You Factorize An Equation
Apr 02, 2025
-
Can Triangle Have 2 Right Angles
Apr 02, 2025
-
Common Factors Of 20 And 25
Apr 02, 2025
-
What Is A Negative Rational Number
Apr 02, 2025
-
According To The Fluid Mosaic Model Of The Cell Membrane
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Does Sulfur Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
