What Is End Point In Titration
Juapaving
Mar 21, 2025 · 6 min read
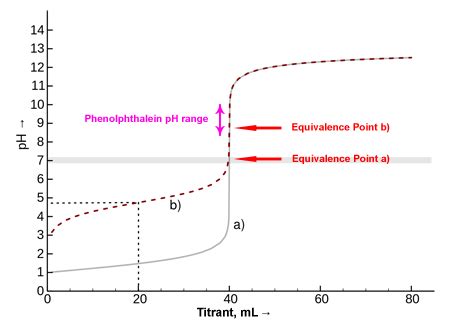
Table of Contents
What is the Endpoint in Titration? A Comprehensive Guide
Titration, a cornerstone technique in analytical chemistry, allows us to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). Understanding the endpoint in titration is crucial for accurate and reliable results. This detailed guide will explore the concept of the endpoint, its significance, factors affecting its determination, and various methods employed to detect it.
Understanding the Endpoint: The Crucial Point in Titration
The endpoint in titration is the point at which the titration is stopped. It's the point at which a noticeable change occurs, signaling that the reaction between the analyte and the titrant is essentially complete. This change is often visually observed through a color change, but it can also be detected using instruments. It's important to differentiate the endpoint from the equivalence point.
Equivalence Point vs. Endpoint: A Key Distinction
The equivalence point is the theoretical point in the titration where the moles of titrant added are stoichiometrically equal to the moles of analyte present. This is the ideal point we aim for, representing the complete neutralization or reaction.
However, the equivalence point is often not directly observable. Instead, we observe the endpoint, which is a close approximation of the equivalence point. The difference between the equivalence point and the endpoint is called the titration error. Minimizing this error is vital for accurate results.
Factors Affecting Endpoint Determination
Several factors can influence the accuracy of endpoint determination, leading to variations in the titration error:
1. Indicator Choice: The Eyes of the Titration
The selection of a suitable indicator is paramount. Indicators are substances that change color depending on the pH or other properties of the solution. The ideal indicator changes color sharply near the equivalence point, minimizing the titration error. The indicator's pKa value, which represents its acidity, is crucial in selecting the appropriate indicator for a specific titration. A pKa value close to the pH at the equivalence point will provide the sharpest color change.
For example, phenolphthalein is a common indicator used in acid-base titrations. It changes color from colorless to pink around pH 8.2. This makes it suitable for titrations where the equivalence point is near this pH, such as the titration of a strong acid with a strong base. However, it's unsuitable for titrations with equivalence points far from pH 8.2.
2. Subjective Observation: The Human Factor
The visual detection of the endpoint, particularly using color change indicators, is subjective and prone to human error. Different observers might perceive the color change at slightly different points, leading to inconsistencies. This is particularly true when the color change is gradual rather than abrupt. To mitigate this, multiple titrations should be performed, and the average result taken.
3. Temperature: A Subtle Influence
Temperature variations can affect the equilibrium of the reaction and the indicator's color change. Significant temperature fluctuations can lead to inaccuracies in endpoint determination. Maintaining a consistent temperature throughout the titration is important for optimal results.
4. Impurities in Solutions: Unexpected Interferences
Impurities in the analyte or titrant solutions can interfere with the reaction and affect the endpoint. These impurities may react with the titrant or interfere with the indicator, leading to deviations from the true equivalence point. Using high-purity reagents is crucial for minimizing this error.
5. Solution Mixing: Ensuring Homogeneity
Inadequate mixing of the solution during the titration can lead to localized concentration variations, resulting in an inaccurate endpoint. Thorough mixing ensures the reaction occurs uniformly throughout the solution, leading to a more precise endpoint.
Methods for Endpoint Detection
While visual color change is the most common method, several other techniques enable endpoint detection, offering higher precision and objectivity:
1. Potentiometric Titration: Electronic Precision
Potentiometric titration employs a pH meter or ion-selective electrode to monitor the change in potential (voltage) during the titration. The equivalence point is determined from the inflection point of the titration curve, a plot of potential versus volume of titrant added. This method offers high accuracy and objectivity, especially when dealing with colorless solutions or weak acids/bases where color changes are subtle.
The potential change near the equivalence point is generally steep, providing a well-defined inflection point for accurate endpoint determination. Software is often used to analyze the titration curve and automatically calculate the endpoint.
2. Conductometric Titration: Measuring Conductivity
Conductometric titration measures the change in the electrical conductivity of the solution during the titration. The equivalence point is determined from the change in the slope of the conductivity curve. This method is particularly useful for titrations involving weak acids or bases, or when the reaction does not involve a significant change in pH.
The conductivity of the solution changes as ions are consumed or produced during the reaction. The change in conductivity near the equivalence point provides a clear indication of the endpoint. This method is less sensitive to indicator choice, overcoming some limitations of visual endpoint determination.
3. Spectrophotometric Titration: Monitoring Absorbance
Spectrophotometric titration monitors the absorbance of the solution at a specific wavelength during the titration. The equivalence point is determined from the change in absorbance at the chosen wavelength. This method is especially useful for titrations involving colored species, as it allows for the quantitative determination of the endpoint.
By monitoring the absorbance, the concentration of a specific reactant or product can be determined as a function of titrant volume. The point of maximum slope in the resulting curve signifies the endpoint. This approach bypasses subjective visual judgments, improving the accuracy and precision of the endpoint determination.
4. Amperometric Titration: Monitoring Current
Amperometric titration measures the change in electric current flowing through the solution during titration. The current is typically measured using an electrode that is sensitive to the presence of a specific ion. The equivalence point is detected as a change in the slope of the current-volume curve. This is especially useful for redox titrations.
The current change often demonstrates a sharp inflection point, providing a clear indication of the endpoint. This method is particularly useful for titrations where other methods may be difficult to apply, such as those with colored solutions or precipitates.
Minimizing Titration Error: Best Practices
Several strategies can significantly reduce the titration error and improve the accuracy of endpoint determination:
- Use appropriate indicators: Select indicators with pKa values close to the expected equivalence point pH.
- Perform multiple titrations: Conduct several titrations to obtain an average result, minimizing the impact of random errors.
- Use standardized solutions: Employ titrants with accurately known concentrations.
- Ensure proper mixing: Mix the solution thoroughly during the titration.
- Control temperature: Maintain a consistent temperature throughout the titration.
- Use appropriate equipment: Employ accurate volumetric glassware and measuring devices.
- Employ alternative endpoint detection methods: Consider using potentiometric, conductometric, spectrophotometric, or amperometric techniques for improved accuracy and objectivity.
Conclusion: Mastering the Endpoint for Accurate Results
The endpoint in titration is a critical point that marks the completion of the reaction between the analyte and the titrant. While visual observation using indicators is a common method, other more objective and precise techniques exist, such as potentiometric, conductometric, spectrophotometric and amperometric titrations. Understanding the factors affecting endpoint determination and employing appropriate techniques are crucial for minimizing titration error and achieving accurate and reliable results in analytical chemistry. By mastering the art of endpoint determination, you can confidently and effectively utilize titration for precise quantitative analysis.
Latest Posts
Latest Posts
-
Which State Of Matter Has A Definite Shape And Volume
Mar 27, 2025
-
What Is The S I Unit For Temperature
Mar 27, 2025
-
Write A Short Note On Apiculture
Mar 27, 2025
-
Dissolving Sugar In Water Is A Chemical Change
Mar 27, 2025
-
Which Of The Following Is An Abstract Word
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is End Point In Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
