Which State Of Matter Has A Definite Shape And Volume
Juapaving
Mar 27, 2025 · 6 min read
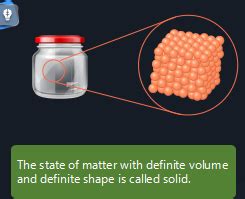
Table of Contents
- Which State Of Matter Has A Definite Shape And Volume
- Table of Contents
- Which State of Matter Has a Definite Shape and Volume? Understanding Solids
- The Defining Characteristics of Solids
- Crystalline vs. Amorphous Solids
- Comparing Solids to Liquids and Gases
- Solids vs. Liquids
- Solids vs. Gases
- The Role of Intermolecular Forces
- Types of Intermolecular Forces
- Examples of Solids and Their Applications
- Conclusion: The Significance of Solid State Matter
- Latest Posts
- Latest Posts
- Related Post
Which State of Matter Has a Definite Shape and Volume? Understanding Solids
The question of which state of matter possesses a definite shape and volume is a fundamental concept in chemistry and physics. The answer, simply put, is the solid state. Unlike liquids and gases, solids exhibit both a fixed shape and a fixed volume, a characteristic stemming from the strong intermolecular forces holding their constituent particles together. Let's delve deeper into the properties of solids, exploring the reasons behind their rigid structure and contrasting them with liquids and gases.
The Defining Characteristics of Solids
Solids are characterized by their strong intermolecular forces, which are the attractive forces between molecules. These forces are significantly stronger in solids compared to liquids and gases. This strong attraction results in the constituent particles – atoms, molecules, or ions – being tightly packed together in a highly ordered arrangement. This ordered structure is what gives solids their definite shape and volume. You can't easily compress a solid into a smaller volume, nor can you easily change its shape without applying considerable force.
Crystalline vs. Amorphous Solids
While all solids share the characteristic of having a definite shape and volume, they can be further classified into two main categories: crystalline and amorphous solids. This classification is based on the arrangement of their constituent particles.
Crystalline solids are characterized by a highly ordered, three-dimensional arrangement of their constituent particles. These particles are arranged in a repeating pattern that extends throughout the entire solid. This regular arrangement gives crystalline solids their characteristic properties, such as sharp melting points and anisotropic properties (meaning their properties vary with direction). Examples of crystalline solids include diamonds (carbon), table salt (sodium chloride), and quartz (silicon dioxide). The regular arrangement also often manifests as visible crystalline structures, leading to the beautiful geometric shapes seen in many minerals.
Amorphous solids, on the other hand, lack this long-range order. Their particles are arranged randomly, similar to liquids, but their strong intermolecular forces prevent them from flowing. This lack of order leads to properties that differ from crystalline solids, such as a gradual softening upon heating rather than a sharp melting point, and isotropic properties (meaning their properties are the same in all directions). Examples of amorphous solids include glass, rubber, and many plastics.
Comparing Solids to Liquids and Gases
To fully appreciate the unique properties of solids, let's compare them to liquids and gases. The differences in their properties arise directly from the strengths of the intermolecular forces and the degree of order among their particles.
Solids vs. Liquids
Liquids have a definite volume but take the shape of their container. This is because the intermolecular forces in liquids are weaker than in solids, allowing the molecules to move around and slide past each other. This movement prevents liquids from maintaining a fixed shape, but their volume remains relatively constant because the molecules are still relatively close together. The contrast between solid and liquid is clearly seen when considering a substance like water: ice (solid) retains a definite shape and volume, while liquid water conforms to the shape of its container.
Solids vs. Gases
Gases, unlike solids and liquids, have neither a definite shape nor a definite volume. Their molecules are far apart and move freely, expanding to fill whatever container they are in. The weak intermolecular forces in gases allow for significant compressibility; you can easily reduce the volume of a gas by applying pressure. The differences are easily observable when you compare a block of ice (solid) to water vapor (gas). The ice maintains its volume and shape, while the vapor expands to fill the entire space available.
The Role of Intermolecular Forces
The strength of intermolecular forces is the key factor in determining the state of matter. These forces are electrostatic attractions between molecules, and their strength varies depending on the type of molecule and its structure.
Types of Intermolecular Forces
Several types of intermolecular forces exist, including:
-
London Dispersion Forces (LDFs): These are the weakest type of intermolecular force and are present in all molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles that induce dipoles in neighboring molecules. LDFs are particularly significant in nonpolar molecules.
-
Dipole-Dipole Forces: These forces occur between polar molecules, which possess permanent dipoles due to differences in electronegativity between atoms. The positive end of one molecule attracts the negative end of another.
-
Hydrogen Bonds: These are a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom such as oxygen, nitrogen, or fluorine. Hydrogen bonds are relatively strong compared to other intermolecular forces.
-
Ionic Bonds: These are strong electrostatic attractions between oppositely charged ions. They are present in ionic compounds like sodium chloride (table salt).
The stronger the intermolecular forces, the more tightly the molecules are held together, resulting in a solid state. Conversely, weaker intermolecular forces lead to liquids or gases. The temperature also plays a crucial role, as increased thermal energy can overcome the intermolecular forces, leading to a phase transition from solid to liquid to gas.
Examples of Solids and Their Applications
Solids are ubiquitous in our daily lives, serving a wide range of purposes. Let's look at a few specific examples:
-
Metals: Metals like iron, copper, and aluminum are crystalline solids known for their high strength, ductility (ability to be drawn into wires), and malleability (ability to be hammered into sheets). They are extensively used in construction, manufacturing, and electronics.
-
Ceramics: Ceramics are inorganic non-metallic solids made from clay minerals. They are known for their hardness, high melting points, and resistance to corrosion. They find applications in various fields, including construction (bricks, tiles), cookware, and electronics (insulators).
-
Polymers: Polymers are large molecules composed of repeating units called monomers. Many polymers are amorphous solids, such as plastics (polyethylene, polypropylene), and rubber. They are widely used in packaging, textiles, and countless other applications.
-
Semiconductors: Semiconductors, like silicon and germanium, are crystalline solids with electrical conductivity intermediate between conductors and insulators. Their unique properties are crucial for the electronics industry, forming the foundation of computer chips and other electronic devices.
Conclusion: The Significance of Solid State Matter
Understanding the properties of solids, particularly their definite shape and volume, is crucial for numerous scientific and technological advancements. From designing stronger and lighter materials to developing advanced electronic devices, our knowledge of solid-state physics and chemistry underpins countless applications. The strong intermolecular forces, the ordered arrangement of particles (in crystalline solids), and the various types of solids showcase the rich diversity within this state of matter. The differences between solids, liquids, and gases, stemming from the relative strength of intermolecular forces, fundamentally shape the world around us. The distinct characteristics of solids allow for the creation of structures, tools, and technologies that are essential to modern life. Further research into the properties and behavior of solids continues to drive innovation across numerous fields.
Latest Posts
Latest Posts
-
How Do You Spell 20 In Word Form
Apr 01, 2025
-
What Does Xxvii Mean In Roman Numerals
Apr 01, 2025
-
Root 2 Root 2 Root 2
Apr 01, 2025
-
How Do You Spell 90 In Word Form
Apr 01, 2025
-
200 Is What Percent Of 50
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
