What Is Difference Between Endothermic And Exothermic
Juapaving
Mar 21, 2025 · 5 min read
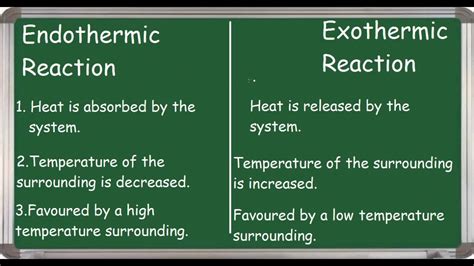
Table of Contents
What's the Difference Between Endothermic and Exothermic Reactions? A Deep Dive
Understanding the difference between endothermic and exothermic reactions is fundamental to grasping many concepts in chemistry and physics. While seemingly simple at first glance, the nuances of these processes, their applications, and their implications for various systems can be surprisingly complex and fascinating. This comprehensive guide will delve into the core principles, providing a detailed comparison of endothermic and exothermic reactions with numerous real-world examples.
Defining Endothermic and Exothermic Reactions: Energy's Role
At the heart of the distinction lies energy transfer. Chemical reactions involve the breaking and forming of chemical bonds. These processes either release or absorb energy in the form of heat. This energy exchange is the defining characteristic that separates endothermic from exothermic reactions.
Exothermic Reactions: Releasing Energy
Exothermic reactions release energy into their surroundings. This energy is typically manifested as heat, causing a noticeable temperature increase in the reaction environment. Think of it like this: the system (the reaction itself) is losing energy, and that energy is transferred to the surroundings. This energy transfer is often described as a negative change in enthalpy (ΔH < 0). The products of an exothermic reaction have lower energy than the reactants.
Key characteristics of exothermic reactions:
- Heat release: A significant temperature increase is observed.
- Negative enthalpy change (ΔH < 0): The system loses energy.
- Spontaneous tendency: Many exothermic reactions occur spontaneously under standard conditions, although spontaneity isn't solely determined by enthalpy.
- Examples: Combustion (burning of fuels), neutralization reactions (acid-base reactions), respiration (biological energy production), and the formation of many ionic compounds.
Endothermic Reactions: Absorbing Energy
In contrast to exothermic processes, endothermic reactions absorb energy from their surroundings. This energy absorption results in a decrease in the temperature of the reaction environment. The system (reaction) is gaining energy, drawing it from its surroundings. This is represented by a positive change in enthalpy (ΔH > 0). The products of an endothermic reaction have higher energy than the reactants.
Key characteristics of endothermic reactions:
- Heat absorption: A noticeable temperature decrease is observed.
- Positive enthalpy change (ΔH > 0): The system gains energy.
- Non-spontaneous tendency (often): Many endothermic reactions require an external energy input to proceed, though some can occur spontaneously under specific conditions.
- Examples: Photosynthesis (plants converting light energy into chemical energy), melting ice, evaporating water, and many decomposition reactions.
Deeper Dive: Enthalpy and Activation Energy
Understanding the concepts of enthalpy and activation energy helps to further clarify the differences between endothermic and exothermic reactions.
Enthalpy (ΔH): The Heat Content
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure. The change in enthalpy (ΔH) during a reaction indicates the amount of heat absorbed or released. A negative ΔH signifies an exothermic reaction (heat released), while a positive ΔH signifies an endothermic reaction (heat absorbed).
Activation Energy (Ea): The Energy Barrier
Activation energy is the minimum amount of energy required for a reaction to occur. Regardless of whether a reaction is endothermic or exothermic, it needs to overcome this energy barrier. This energy is used to break existing bonds before new ones can form. Once the activation energy is overcome, the reaction can proceed, either releasing (exothermic) or absorbing (endothermic) energy overall.
Visualizing Endothermic and Exothermic Reactions: Energy Diagrams
Energy diagrams are helpful visual tools for understanding the energy changes involved in chemical reactions. These diagrams plot the potential energy of the system against the reaction progress.
Exothermic Reaction Diagram: The energy of the products is lower than the energy of the reactants, indicating a net release of energy. The difference between the reactant energy and product energy represents the ΔH (negative value). The "hill" represents the activation energy (Ea) that must be overcome for the reaction to proceed.
Endothermic Reaction Diagram: The energy of the products is higher than the energy of the reactants, indicating a net absorption of energy. The difference between reactant and product energy represents the ΔH (positive value). Again, the "hill" represents the activation energy (Ea).
Real-World Examples: Illuminating the Differences
Let's look at some concrete examples to solidify our understanding.
Exothermic Reactions: Abundant in Everyday Life
- Combustion: Burning wood, gas, or other fuels is a classic example. The heat and light released are evidence of the energy released during the oxidation process.
- Neutralization Reactions: When an acid reacts with a base, the reaction releases heat. This is why mixing strong acids and bases can be dangerous—the heat generated can be significant.
- Respiration: Our bodies constantly undergo exothermic reactions to produce energy from food. This energy powers our bodily functions.
- Explosions: Many explosions are driven by extremely rapid exothermic reactions that release a massive amount of energy in a short period.
Endothermic Reactions: Less Obvious, but Equally Important
- Photosynthesis: Plants absorb sunlight energy to convert carbon dioxide and water into glucose and oxygen. This process requires energy input and is thus endothermic.
- Melting Ice: To melt ice, heat energy must be absorbed to break the bonds holding the water molecules together in a solid structure.
- Evaporating Water: Similar to melting ice, converting liquid water to vapor requires energy input to overcome intermolecular forces.
- Cooking an Egg: While seemingly straightforward, cooking an egg involves a complex series of endothermic reactions as the proteins denature and change structure.
Practical Applications: Harnessing the Power of Energy Transfer
The understanding and application of endothermic and exothermic reactions are crucial in many fields:
- Industry: Exothermic reactions are utilized in various industrial processes, including power generation, manufacturing of materials, and chemical synthesis. Endothermic reactions find applications in areas like refrigeration and air conditioning.
- Medicine: Many medical treatments and diagnostic techniques rely on the principles of energy transfer in chemical reactions.
- Environmental Science: Understanding endothermic and exothermic reactions is crucial for assessing the environmental impact of various processes and designing sustainable solutions.
Conclusion: A Fundamental Concept with Far-Reaching Implications
The distinction between endothermic and exothermic reactions is a fundamental concept in chemistry and physics with wide-ranging implications across various fields. By understanding the energy changes involved in these processes, we can better comprehend how energy is transferred and utilized in natural and engineered systems. From the simple act of lighting a match to the complex processes of life itself, endothermic and exothermic reactions are integral parts of the world around us. Further exploration of thermodynamics will provide even deeper insights into these fascinating phenomena and their role in shaping our universe.
Latest Posts
Latest Posts
-
The Demand Curve For A Monopolist Is
Mar 21, 2025
-
Examples Of Complete And Incomplete Metamorphosis
Mar 21, 2025
-
What Are The Prime Factors Of 160
Mar 21, 2025
-
Definition Of Rotational Motion In Physics
Mar 21, 2025
-
What Is The Lcm Of 2 4 5
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is Difference Between Endothermic And Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
