What Is A Row In The Periodic Table
Juapaving
Mar 21, 2025 · 6 min read
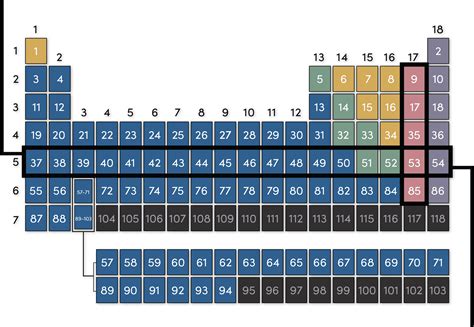
Table of Contents
What is a Row in the Periodic Table? Understanding Periods and Their Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While columns, known as groups, represent elements sharing similar chemical characteristics, rows, or periods, reveal a different story – the gradual filling of electron shells and the resulting trends in atomic size, ionization energy, and electronegativity. This comprehensive guide delves into the intricacies of rows in the periodic table, explaining their significance, the underlying principles that govern their arrangement, and the fascinating patterns they reveal about the behavior of elements.
Understanding the Structure of a Row
Each row in the periodic table corresponds to a principal energy level, or shell, in an atom. As you move across a period from left to right, the number of protons and electrons increases, gradually filling the electron shells. The first row, for instance, contains only hydrogen (H) and helium (He), because they only require a single energy level to accommodate their electrons. The second row, including lithium (Li) to neon (Ne), represents the filling of the second principal energy level. This systematic progression continues across the subsequent rows, with each period adding another principal energy level.
The Significance of Electron Shells
The significance of electron shells lies in their influence on the chemical properties of elements. The outermost shell, called the valence shell, holds the valence electrons. These electrons are crucial for chemical bonding, dictating how atoms interact and form molecules. The number of valence electrons generally increases as you progress across a period. This change in the number of valence electrons directly impacts the reactivity and bonding capabilities of elements within the same row.
Periodic Trends Across a Row: A Closer Look
Several key periodic trends manifest themselves as we move across a row in the periodic table. These trends offer valuable insights into the behavior of elements and help predict their chemical properties.
1. Atomic Radius: Decreasing Across a Period
Atomic radius refers to the size of an atom. As you move from left to right across a period, the atomic radius generally decreases. This is because, although an additional electron shell is being filled, the increasing nuclear charge (number of protons) exerts a stronger attractive force on the electrons, pulling them closer to the nucleus. This stronger pull overcomes the repulsive forces between electrons, resulting in a smaller atomic size.
Exceptions and Considerations:
While the general trend is a decrease in atomic radius, there are exceptions, particularly among transition metals. The complexities of electron shielding and orbital interactions can cause slight variations in this trend. These variations emphasize the need for a deeper understanding of atomic structure and electron configurations beyond simply counting electrons.
2. Ionization Energy: Increasing Across a Period
Ionization energy represents the energy required to remove an electron from a gaseous atom. As you move across a period, ionization energy generally increases. This is a direct consequence of the increasing nuclear charge. The stronger attraction between the nucleus and the electrons makes it increasingly difficult to remove an electron, requiring more energy.
Analyzing the Trend:
The consistent increase in ionization energy underscores the enhanced stability of atoms with filled or half-filled subshells. Elements with a full valence shell (noble gases) exhibit exceptionally high ionization energies, demonstrating their remarkable chemical inertness. This concept provides a foundational understanding of chemical reactivity and stability.
3. Electronegativity: Increasing Across a Period
Electronegativity measures an atom's ability to attract electrons towards itself in a chemical bond. Similar to ionization energy, electronegativity generally increases across a period. The increasing nuclear charge exerts a stronger pull on bonding electrons, increasing the atom's ability to attract electrons from other atoms.
Bond Polarity and Electronegativity:
The difference in electronegativity between two atoms determines the polarity of a chemical bond. A large difference signifies a polar covalent bond, where electrons are unequally shared. Understanding electronegativity is crucial in predicting the nature of chemical bonds and the properties of resulting molecules.
4. Metallic Character: Decreasing Across a Period
Metallic character refers to the properties associated with metals, such as conductivity, malleability, and ductility. As you move across a period, metallic character generally decreases. This is because, as the nuclear charge increases and the atomic radius decreases, atoms tend to hold onto their electrons more tightly, reducing their tendency to lose electrons and form positive ions (cations), a characteristic property of metals.
Nonmetals and Their Properties:
The decrease in metallic character corresponds to an increase in non-metallic character. Nonmetals generally have high ionization energies, high electronegativities, and tend to gain electrons to form negative ions (anions). The transition from metallic to non-metallic behavior provides a clear distinction in chemical reactivity and bonding preferences.
Specific Examples of Periodic Trends within Rows
Let's explore specific examples within the rows to further illustrate these trends:
Period 2 (Lithium to Neon):
- Lithium (Li): Highly reactive metal, readily loses its one valence electron. Low ionization energy and electronegativity.
- Beryllium (Be): Less reactive than lithium, still exhibits metallic properties. Higher ionization energy and electronegativity than lithium.
- Boron (B): Metalloid, showing intermediate properties between metals and nonmetals.
- Carbon (C): Nonmetal, can form strong covalent bonds. Higher electronegativity than boron.
- Nitrogen (N): Nonmetal, forms strong triple bonds with itself. High electronegativity.
- Oxygen (O): Nonmetal, highly reactive, readily forms double bonds. High electronegativity.
- Fluorine (F): Nonmetal, extremely reactive, highest electronegativity in this period.
- Neon (Ne): Noble gas, highly unreactive due to its full valence shell. Extremely high ionization energy.
Period 3 (Sodium to Argon):
Similar trends are observed in Period 3, with sodium (Na) exhibiting strong metallic character and argon (Ar) being a chemically inert noble gas. The gradual change in properties across this row demonstrates the periodic nature of elemental characteristics. The larger atomic size of elements in Period 3 compared to Period 2 leads to slight variations in the magnitude of the periodic trends, but the overall direction remains consistent.
The Importance of Understanding Row Trends
Understanding the trends across rows in the periodic table is crucial for several reasons:
- Predicting Chemical Properties: The trends allow for the prediction of the chemical properties of elements based on their position in the table.
- Explaining Chemical Reactivity: They explain why certain elements are more reactive than others and how they interact with each other.
- Designing New Materials: The understanding of periodic trends is paramount in the design and synthesis of new materials with specific properties, such as superconductors or high-strength alloys.
- Analyzing Chemical Reactions: They are essential for understanding the course and products of chemical reactions.
- Developing New Technologies: Knowledge of these trends plays a key role in the development of new technologies.
Conclusion: The Continuing Story of the Periodic Table
The rows in the periodic table represent a fundamental aspect of atomic structure and its influence on elemental properties. The systematic increase in atomic number across each row leads to predictable trends in atomic radius, ionization energy, electronegativity, and metallic character. These trends, while showing general patterns, exhibit exceptions and nuances that challenge and refine our understanding of atomic behavior. Continued exploration and study of these trends are crucial for advancements in chemistry and related fields. Understanding the rows, in conjunction with understanding the columns or groups, provides a complete picture of the fascinating world of chemical elements and their interactions. The periodic table remains a powerful tool, constantly providing new insights and driving innovation across diverse scientific disciplines.
Latest Posts
Latest Posts
-
What Are Examples Of Unit Rates
Mar 27, 2025
-
What Is The Least Common Multiple Of 18 And 6
Mar 27, 2025
-
Which State Of Matter Has A Definite Shape And Volume
Mar 27, 2025
-
What Is The S I Unit For Temperature
Mar 27, 2025
-
Write A Short Note On Apiculture
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is A Row In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
