What Is A Positively Charged Ion Called
Juapaving
Apr 05, 2025 · 6 min read
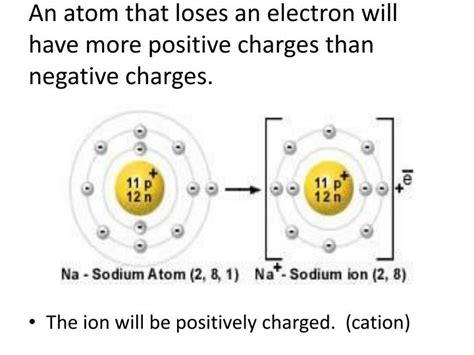
Table of Contents
What is a Positively Charged Ion Called? A Deep Dive into Cations
A positively charged ion, often referred to as a cation, plays a crucial role in various chemical and biological processes. Understanding its properties, formation, and significance is essential in various scientific fields. This comprehensive article will delve into the intricacies of cations, exploring their definition, formation mechanisms, nomenclature, properties, and applications across different disciplines.
Understanding Ions: The Foundation of Cations
Before diving into the specifics of cations, it's crucial to establish a foundational understanding of ions in general. Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net electrical charge. This charge imbalance is what distinguishes ions from neutral atoms or molecules. The process of gaining or losing electrons is called ionization.
There are two primary types of ions:
- Cations: Positively charged ions formed when an atom loses one or more electrons.
- Anions: Negatively charged ions formed when an atom gains one or more electrons.
The Formation of Cations: A Closer Look
The formation of a cation is a fundamental concept in chemistry. It typically occurs when an atom with a relatively low ionization energy loses one or more electrons. This ionization energy, often measured in electron volts (eV), represents the energy required to remove an electron from a neutral atom. Atoms with lower ionization energies are more likely to form cations.
Several factors influence the ease with which an atom forms a cation:
- Atomic radius: Smaller atoms generally have higher ionization energies, making them less likely to lose electrons. Conversely, larger atoms are more prone to cation formation.
- Nuclear charge: A higher nuclear charge (more protons) increases the attractive force on electrons, making it harder to remove them and thus less likely to form a cation.
- Electron shielding: Inner electrons shield outer electrons from the full nuclear charge. Greater shielding reduces the effective nuclear charge experienced by outer electrons, making them easier to remove and more prone to cation formation.
- Electron configuration: Atoms tend to lose electrons to achieve a stable electron configuration, often a filled valence shell (like noble gases). This drive for stability is a major driving force behind cation formation.
Examples of Cation Formation
Let's illustrate cation formation with a few examples:
- Sodium (Na): Sodium has one electron in its outermost shell. It readily loses this electron to achieve a stable octet configuration, forming a sodium cation (Na⁺).
- Calcium (Ca): Calcium has two electrons in its outermost shell. It loses these two electrons to form a calcium cation (Ca²⁺).
- Aluminum (Al): Aluminum has three valence electrons and readily loses them to form an aluminum cation (Al³⁺).
Nomenclature of Cations: Naming Positively Charged Ions
Naming cations follows relatively straightforward rules:
-
Monatomic Cations: For single-atom cations (monatomic cations), the name of the cation is simply the name of the element followed by the word "ion" or, more commonly, indicated with a positive charge superscript (e.g., Na⁺, Ca²⁺, Al³⁺). For transition metals that can form multiple cations (e.g., iron, copper), Roman numerals are used in parentheses after the element name to specify the charge (e.g., Iron(II) ion, Fe²⁺; Iron(III) ion, Fe³⁺).
-
Polyatomic Cations: Polyatomic cations are groups of atoms that carry a positive charge. These are often named using specific conventions based on their constituent elements or functional groups. For instance, the ammonium ion (NH₄⁺) is a common example of a polyatomic cation.
Properties of Cations: A Closer Examination
Cations exhibit several key properties that distinguish them from neutral atoms and anions:
- Positive Charge: The defining characteristic of a cation is its positive electrical charge, stemming from the loss of electrons.
- Smaller Size: Compared to their parent atoms, cations are generally smaller due to the loss of electrons and the increased effective nuclear charge. This reduction in size significantly impacts their reactivity and interactions with other species.
- High Reactivity: Cations are highly reactive, especially those with higher charges. Their positive charge attracts negatively charged species (anions or electrons), leading to various chemical reactions and formations of ionic compounds.
- Electrostatic Interactions: Cations strongly interact with anions through electrostatic forces, forming ionic bonds that stabilize ionic compounds (like salts). The strength of these interactions depends on the magnitude of the charges and the distance between the ions.
Applications of Cations: A Wide Spectrum of Uses
Cations play vital roles in a wide range of applications across diverse scientific and technological domains:
-
Biology: Cations, such as sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺), are essential for numerous biological processes. They are involved in nerve impulse transmission, muscle contraction, enzyme activity, and maintaining osmotic balance within cells.
-
Chemistry: Cations are fundamental building blocks in chemical compounds. They form ionic bonds with anions, leading to the formation of salts and other ionic compounds, which have diverse applications in various industries. Cationic reactions are essential to many chemical processes.
-
Materials Science: Cations are integral in the development and design of new materials. Their properties influence the physical and chemical characteristics of materials, particularly in ceramics, glasses, and advanced composites.
-
Medicine: Cations have crucial roles in medicine. Electrolyte balance, involving the regulation of cations like sodium, potassium, and calcium, is critical for maintaining proper physiological function. Imbalances can lead to various medical conditions.
-
Environmental Science: Cations play crucial roles in environmental processes, particularly in water chemistry and soil science. The presence and concentration of specific cations impact water quality, nutrient cycling, and soil fertility.
-
Industrial Applications: Numerous industrial processes rely on cations. They are vital components in batteries, catalysts, and various other industrial applications.
Beyond the Basics: Exploring Complex Cationic Systems
While the fundamental aspects of cations are relatively straightforward, more complex systems involving cations present fascinating challenges and research opportunities. For instance:
-
Transition Metal Cations: Transition metal cations, which exhibit variable oxidation states, demonstrate complex behavior due to the involvement of d-electrons in bonding and reactivity. Their coordination chemistry is a significant area of research, impacting catalysis, materials science, and biological systems.
-
Organometallic Cations: Organometallic cations, which contain both carbon and metal atoms, have become increasingly important in catalysis and organic synthesis. Their unique bonding properties lead to distinct reactivity patterns, allowing the efficient preparation of complex organic molecules.
-
Cationic Polymers: Cationic polymers possess positively charged functional groups along their backbone, which influences their interactions with various materials. They find applications in drug delivery, flocculation, and surface modification.
Conclusion: The Ubiquitous Role of Cations
In conclusion, the positively charged ion, the cation, is a fundamental chemical entity with a profound impact across various scientific disciplines. Understanding its formation, properties, and applications is essential for advancements in chemistry, biology, materials science, and many other fields. From the biological processes maintaining life to the design of advanced materials, the role of cations is far-reaching and continues to be a subject of ongoing research and innovation. Its significance underscores the importance of continued investigation into the fascinating world of ionic chemistry.
Latest Posts
Latest Posts
-
50 Yards Is How Many Feet
Apr 05, 2025
-
Bronsted Lowry Vs Lewis Vs Arrhenius
Apr 05, 2025
-
Name Two Constituents Of Baking Powder
Apr 05, 2025
-
The Study Of Tissue Is Known As
Apr 05, 2025
-
5 Is What Percent Of 8
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about What Is A Positively Charged Ion Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
