What Happens When Egg Shell Is Added To Nitric Acid
Juapaving
Mar 16, 2025 · 5 min read
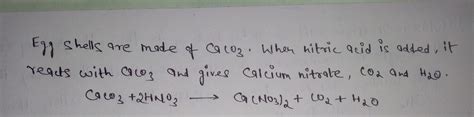
Table of Contents
What Happens When Eggshells Are Added to Nitric Acid? A Comprehensive Exploration
The seemingly simple act of adding eggshells to nitric acid unleashes a fascinating chemical reaction, revealing the underlying composition of eggshells and the potent nature of nitric acid. This process isn't just a visually striking experiment; it offers a deeper understanding of chemistry principles, including acid-base reactions, redox reactions, and the formation of various compounds. This article will delve into the intricacies of this reaction, exploring the chemical processes involved, the safety precautions necessary, and the potential applications of the resulting products.
Understanding the Reactants: Eggshells and Nitric Acid
Before examining the reaction, let's understand the individual components:
Eggshells: A Calcium Carbonate Reservoir
Eggshells are primarily composed of calcium carbonate (CaCO₃), a naturally occurring compound also known as calcite. This accounts for approximately 95% of the eggshell's mass. The remaining 5% consists of other minerals, including magnesium carbonate, calcium phosphate, and various proteins. The crystalline structure of calcium carbonate gives eggshells their strength and protective properties. It's this calcium carbonate that will be the primary reactant in our reaction with nitric acid.
Nitric Acid: A Strong Oxidizing Acid
Nitric acid (HNO₃) is a highly corrosive and strong oxidizing acid. Its oxidizing nature means it readily accepts electrons from other substances, leading to redox reactions. This property is crucial in understanding the reaction with calcium carbonate. Nitric acid's strength also means it readily reacts with bases, making its interaction with calcium carbonate (a base) highly vigorous. The concentration of nitric acid significantly impacts the reaction's speed and outcome. Concentrated nitric acid reacts much more aggressively than dilute nitric acid.
The Reaction: Dissolution and Beyond
When eggshells are added to nitric acid, a multi-step reaction occurs. The primary reaction involves the acid-base reaction between nitric acid and calcium carbonate:
CaCO₃(s) + 2HNO₃(aq) → Ca(NO₃)₂(aq) + H₂O(l) + CO₂(g)
This equation depicts the following:
- CaCO₃(s): Solid calcium carbonate (eggshell).
- 2HNO₃(aq): Two moles of aqueous nitric acid.
- Ca(NO₃)₂(aq): Aqueous calcium nitrate, a soluble salt.
- H₂O(l): Liquid water.
- CO₂(g): Gaseous carbon dioxide, responsible for the effervescence.
This reaction is characterized by vigorous effervescence, the release of carbon dioxide gas, which you will see as bubbles forming and escaping from the solution. The reaction is exothermic, meaning it releases heat. The solution will warm up noticeably.
Beyond the Primary Reaction: The Role of Nitric Acid's Oxidizing Power
While the above equation captures the main reaction, the powerful oxidizing properties of nitric acid come into play, particularly with concentrated nitric acid. The oxidizing nature of nitric acid can lead to the formation of nitrogen oxides (NOₓ), depending on the concentration of the acid and the reaction conditions. These oxides are typically brown or reddish-brown gases and contribute to the characteristic fumes observed during the reaction.
The formation of nitrogen oxides is a redox reaction, where nitric acid is reduced (gains electrons) while some components of the eggshell might undergo oxidation (lose electrons). This side reaction adds complexity to the overall process and generates potentially harmful gases.
Observing the Reaction: A Step-by-Step Guide (Simulated, Do Not Perform This Experiment Without Proper Safety Precautions)
While performing this experiment at home is strongly discouraged due to safety hazards, understanding the visual changes is important for conceptualizing the reaction:
- Initial Observation: Adding eggshells to nitric acid will immediately result in vigorous bubbling (effervescence) due to the release of carbon dioxide gas.
- Solution Changes: The solution will gradually become clearer as the calcium carbonate dissolves. The initial milky appearance will diminish as the calcium carbonate is converted into soluble calcium nitrate.
- Gas Evolution: A significant amount of carbon dioxide gas will be released, and depending on the concentration of the nitric acid, brown or reddish-brown nitrogen oxide gases might also be observed.
- Temperature Change: The reaction is exothermic, leading to a noticeable increase in the solution's temperature.
- Final Solution: Once the reaction subsides, the remaining solution will primarily contain calcium nitrate, water, and possibly small amounts of other dissolved minerals from the eggshell.
Safety Precautions: Handling Nitric Acid and its Reaction Products
Crucially, conducting this experiment at home is extremely dangerous and should not be attempted without proper laboratory equipment and training. Nitric acid is a highly corrosive substance, and the reaction generates toxic gases. The following precautions are essential in a laboratory setting:
- Eye Protection: Safety goggles are mandatory. Nitric acid is extremely damaging to eyes.
- Protective Clothing: A lab coat, gloves made of appropriate resistant material (nitrile or neoprene), and closed-toe shoes should be worn.
- Ventilation: The reaction should be performed under a well-ventilated fume hood to prevent inhalation of toxic gases like nitrogen oxides.
- Waste Disposal: Nitric acid and its reaction products require careful disposal according to local regulations. Never pour them down the drain.
- First Aid: Have access to appropriate first aid materials and know the emergency procedures for chemical spills and exposure.
Applications and Further Exploration
While the reaction primarily serves as a demonstration of chemical principles, the resulting calcium nitrate has several applications:
- Fertilizers: Calcium nitrate is a valuable source of nitrogen and calcium for plants, making it a common ingredient in fertilizers.
- Food Preservation: It can act as a food preservative, preventing bacterial growth.
- Other Chemical Processes: It serves as a precursor in various other chemical processes.
Conclusion: A Chemical Reaction Rich in Learning
The reaction between eggshells and nitric acid is a visually striking and conceptually rich chemical process. It showcases the principles of acid-base reactions, redox reactions, and the importance of safety in handling corrosive chemicals. While performing this reaction at home is strongly discouraged due to the inherent risks, understanding the underlying chemistry provides a valuable insight into the composition of everyday materials and the powerful reactivity of strong acids. This reaction serves as a potent example of the fascinating world of chemical transformations and the importance of responsible experimentation.
Latest Posts
Latest Posts
-
Write 50 As A Product Of Prime Factors
Mar 16, 2025
-
What Is Found In Both Dna And Rna
Mar 16, 2025
-
Rotational Symmetry Letters Of The Alphabet
Mar 16, 2025
-
What Is The Outermost Layer Of The Sun Called
Mar 16, 2025
-
What Are The Common Factors Of 25
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Happens When Egg Shell Is Added To Nitric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
