What Happens To A Cell In A Hypertonic Solution
Juapaving
Mar 27, 2025 · 5 min read
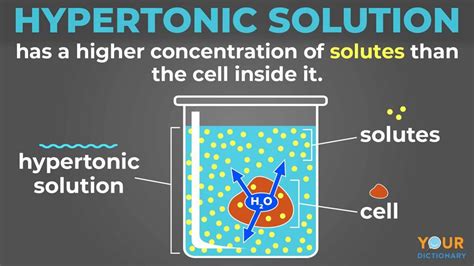
Table of Contents
What Happens to a Cell in a Hypertonic Solution? A Deep Dive into Osmosis and Cell Response
Understanding how cells react to different environments is fundamental to biology. One crucial concept is osmosis, the movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. A key scenario in understanding osmosis involves placing a cell in a hypertonic solution. This article delves deep into what happens to a cell in a hypertonic solution, exploring the underlying mechanisms, the consequences for different cell types, and the broader implications for biological systems.
Understanding Hypertonic Solutions and Osmosis
Before exploring the cellular effects, let's define our terms. A hypertonic solution is one in which the concentration of solutes (dissolved substances) is higher outside the cell than inside. This means the water concentration is lower outside the cell. Conversely, a hypotonic solution has a lower solute concentration outside the cell, and an isotonic solution has equal solute concentrations inside and outside the cell.
Osmosis, as mentioned, is the passive movement of water across a selectively permeable membrane. This membrane, typically a cell's plasma membrane, allows water to pass through but restricts the passage of many solutes. Water moves to equalize the concentration of solutes on both sides of the membrane. In a hypertonic environment, water moves out of the cell to try and dilute the higher solute concentration outside.
The Cellular Response: Plasmolysis and its Stages
When a cell is placed in a hypertonic solution, the process of plasmolysis occurs. This refers to the shrinkage of the cytoplasm away from the cell wall, due to water loss. This process unfolds in distinct stages:
Stage 1: Initial Water Loss and Membrane Detachment
The first noticeable change is the gradual loss of water from the cell. As water exits the cell via osmosis, the cell's internal pressure, called turgor pressure, begins to decrease. The cell membrane, no longer fully supported by the turgor pressure, starts to detach from the cell wall. This detachment is particularly evident in plant cells, which possess a rigid cell wall.
Stage 2: Cytoplasm Shrinkage and Increased Solute Concentration
As water continues to leave the cell, the cytoplasm shrinks further. This shrinkage concentrates the solutes within the cell, leading to a progressively higher internal solute concentration. However, this concentration increase is limited, because the amount of solute inside the cell remains constant. The decrease in cell volume and the increase in solute concentration inside are the main hallmarks of this stage.
Stage 3: Plasmolysis Completion and Potential Cell Damage
The process culminates in complete plasmolysis. The cell membrane pulls away from the cell wall significantly, leaving a gap between the two. In severe cases, the plasma membrane can become heavily distorted. This can lead to irreversible damage to the cell's structure and function, potentially resulting in cell death. The extent of damage depends on the duration of exposure to the hypertonic solution and the cell's inherent resilience.
Differences in Response: Plant Cells vs. Animal Cells
The response to a hypertonic solution differs significantly between plant and animal cells, primarily due to the presence of a cell wall in plant cells.
Plant Cells: Plasmolysis and Potential Recovery
In plant cells, the rigid cell wall prevents the cell from completely collapsing. The plasma membrane pulls away from the cell wall, causing the cytoplasm to shrink. This is clearly visible under a microscope. However, if the cell is returned to an isotonic or hypotonic solution, it can often recover from plasmolysis and regain its turgor pressure. The cell wall provides structural support and prevents the cell from lysing (bursting).
Animal Cells: Crenation and Irreversible Damage
Animal cells lack a rigid cell wall. When placed in a hypertonic solution, they undergo crenation, shrinking significantly and potentially becoming severely deformed. The loss of water causes the cell membrane to wrinkle, and the cell may become non-functional or even die. Crenation is often irreversible, as the cell's structural integrity is compromised.
Factors Influencing Plasmolysis and Crenation
Several factors influence the rate and severity of plasmolysis and crenation:
-
Concentration Gradient: A steeper concentration gradient (larger difference in solute concentration across the membrane) leads to faster water movement and more pronounced plasmolysis or crenation.
-
Membrane Permeability: The permeability of the cell membrane to water and solutes affects the rate of osmosis. A more permeable membrane allows for faster water movement.
-
Duration of Exposure: Prolonged exposure to a hypertonic solution results in more severe effects, increasing the likelihood of irreversible damage.
-
Cell Type and Size: Different cell types exhibit varying degrees of sensitivity to hypertonic environments. Smaller cells may be more susceptible to rapid dehydration.
-
Temperature: Temperature influences the rate of osmosis, with higher temperatures generally leading to faster water movement.
Biological Significance and Applications
Understanding the effects of hypertonic solutions has significant implications across various biological fields:
-
Food Preservation: Hypertonic solutions are used in food preservation techniques like salting and sugaring, creating an environment where microorganisms cannot thrive due to water loss.
-
Medicine: Osmosis plays a critical role in intravenous fluid therapy. The concentration of fluids administered must be carefully controlled to avoid damaging red blood cells.
-
Plant Physiology: Understanding plasmolysis is essential for studying plant water relations, drought tolerance, and the effects of salinity on plant growth.
-
Cell Biology Research: Hypertonic solutions are used in experimental settings to study cellular responses to osmotic stress and to manipulate cell volume.
Conclusion: A Complex Cellular Response
The response of a cell to a hypertonic solution, whether plasmolysis in plant cells or crenation in animal cells, is a complex process driven by osmosis. The extent of the effects depends on a variety of factors, including the concentration gradient, membrane permeability, duration of exposure, and cell type. Understanding these cellular responses is crucial for numerous applications in biology, medicine, and agriculture. Further research continues to uncover the intricacies of cellular osmotic regulation and its importance for maintaining cell viability and function. From the preservation of food to the study of plant stress responses, understanding osmosis and its effects on cells is of vital importance across numerous scientific disciplines.
Latest Posts
Latest Posts
-
Examples Of Newtons First Law Of Motion In Everyday Life
Mar 30, 2025
-
What Is The Factors Of 88
Mar 30, 2025
-
In An Endothermic Reaction Energy Is
Mar 30, 2025
-
The Numerical Ratio Of Average Velocity To Average Speed Is
Mar 30, 2025
-
Which Of The Following Is A Vector
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Happens To A Cell In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
