What Does A Negative Delta H Mean
Juapaving
Mar 14, 2025 · 6 min read
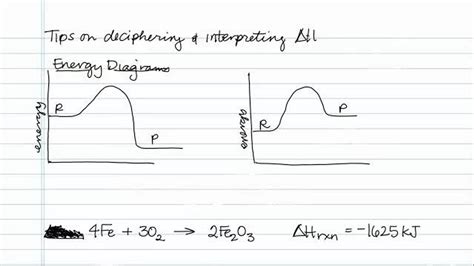
Table of Contents
What Does a Negative ΔH Mean? Understanding Enthalpy Change
Understanding enthalpy change, represented by ΔH, is crucial for comprehending various chemical and physical processes. A negative ΔH value signifies an exothermic reaction or process, indicating a release of heat to the surroundings. This seemingly simple concept has profound implications across numerous scientific fields, from everyday phenomena to complex industrial applications. This comprehensive guide will delve into the meaning of a negative ΔH, explore its significance in different contexts, and provide practical examples to solidify your understanding.
Understanding Enthalpy (H) and Enthalpy Change (ΔH)
Before exploring the implications of a negative ΔH, it's essential to define enthalpy and its change. Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. It's a state function, meaning its value depends only on the system's current state and not on the path taken to reach that state. This makes enthalpy calculations straightforward, focusing on the initial and final states.
Enthalpy change (ΔH), on the other hand, quantifies the heat exchanged between a system and its surroundings during a process at constant pressure. It's calculated as the difference between the final enthalpy (H<sub>final</sub>) and the initial enthalpy (H<sub>initial</sub>):
ΔH = H<sub>final</sub> - H<sub>initial</sub>
A positive ΔH indicates an endothermic reaction, where the system absorbs heat from its surroundings, resulting in a net increase in the system's enthalpy. Conversely, a negative ΔH signifies an exothermic reaction, where the system releases heat to the surroundings, leading to a decrease in the system's enthalpy.
The Significance of a Negative ΔH: Exothermic Reactions
A negative ΔH is the hallmark of an exothermic reaction. This means the reaction releases energy in the form of heat, typically causing a temperature increase in the surroundings. The magnitude of the negative ΔH value represents the amount of heat released. A larger negative value indicates a greater release of heat. These reactions are often spontaneous under standard conditions, though spontaneity is also governed by entropy (ΔS).
Examples of Exothermic Reactions:
-
Combustion: The burning of fuels like wood, propane, or gasoline is a classic example. These reactions release a significant amount of heat, making them valuable energy sources. The negative ΔH values are substantial, driving the combustion process.
-
Neutralization Reactions: The reaction between an acid and a base, forming salt and water, is usually exothermic. The heat released during the neutralization process can be substantial, especially with strong acids and bases.
-
Formation of Ionic Compounds: The formation of many ionic compounds from their constituent ions releases energy, resulting in a negative ΔH. The strong electrostatic attraction between the ions stabilizes the system, releasing energy as heat.
-
Nuclear Reactions (Fission and Fusion): Nuclear fission (splitting atoms) and fusion (combining atoms) release enormous amounts of energy, far exceeding the energy released in chemical reactions. The negative ΔH in these processes is exceptionally large, making them powerful energy sources but also potentially hazardous.
Applications of Exothermic Reactions (Negative ΔH)
The practical applications of exothermic reactions are vast and impact various aspects of modern life:
-
Energy Production: Power plants utilize the exothermic combustion of fossil fuels (coal, oil, natural gas) to generate electricity. Nuclear power plants exploit the immense exothermic energy released during nuclear fission.
-
Industrial Processes: Many industrial processes rely on exothermic reactions to drive chemical transformations. For instance, the production of ammonia (Haber-Bosch process) is exothermic and crucial for fertilizer production.
-
Heating and Cooking: The combustion of fuels in stoves and furnaces provides heat for cooking and home heating, taking advantage of exothermic processes.
-
Hand Warmers: Commercial hand warmers utilize the exothermic oxidation of iron to generate heat, providing warmth in cold environments. The reaction is carefully controlled to ensure a sustained and safe release of heat.
Understanding the Relationship Between ΔH and Spontaneity
While a negative ΔH strongly suggests spontaneity (the reaction will proceed without external intervention), it's not the sole determinant. Gibbs Free Energy (ΔG), which considers both enthalpy (ΔH) and entropy (ΔS), provides a more complete picture of spontaneity:
ΔG = ΔH - TΔS
where T is the temperature in Kelvin.
- ΔG < 0: The reaction is spontaneous.
- ΔG > 0: The reaction is non-spontaneous.
- ΔG = 0: The reaction is at equilibrium.
A negative ΔH favors spontaneity, but a positive ΔS (increase in disorder) also contributes to spontaneity. At high temperatures, the TΔS term can outweigh a positive ΔH, leading to a spontaneous reaction even if ΔH is positive. Conversely, a large negative ΔH can overcome a negative ΔS at lower temperatures, resulting in spontaneous exothermic reactions.
Factors Affecting the Magnitude of ΔH
Several factors influence the magnitude of the enthalpy change (ΔH) in a reaction:
-
Bond Energies: The strength of chemical bonds plays a significant role. Reactions forming stronger bonds generally release more energy (more negative ΔH).
-
Reaction Stoichiometry: The quantities of reactants involved affect the total heat released or absorbed. Larger amounts of reactants lead to proportionally larger ΔH values.
-
State of Matter: The physical states (solid, liquid, gas) of reactants and products can influence ΔH. Phase transitions (like melting or boiling) contribute to the overall enthalpy change.
-
Temperature and Pressure: While ΔH is typically considered at standard conditions (298K and 1 atm), changes in temperature and pressure can subtly alter the enthalpy change.
Practical Examples and Calculations
Let's consider a simple example: the combustion of methane (CH₄):
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) ΔH = -890 kJ/mol
This equation indicates that burning one mole of methane releases 890 kJ of heat. The negative sign signifies an exothermic reaction. This large negative ΔH explains why methane is a potent fuel source.
To further illustrate, consider the dissolution of sodium hydroxide (NaOH) in water:
NaOH(s) → Na⁺(aq) + OH⁻(aq) ΔH = -44.5 kJ/mol
This reaction also releases heat, evident in the temperature increase observed when dissolving NaOH in water. The negative ΔH confirms the exothermic nature of this dissolution process.
These examples highlight how negative ΔH values provide quantitative information about the heat released during a chemical or physical process.
Conclusion: The Importance of Understanding Negative ΔH
Understanding what a negative ΔH means is fundamental to grasping the principles of thermodynamics and their relevance across various scientific and engineering disciplines. A negative ΔH indicates an exothermic reaction, signifying the release of heat to the surroundings. This seemingly simple concept has far-reaching implications, influencing energy production, industrial processes, and numerous everyday phenomena. By understanding the interplay between enthalpy, entropy, and Gibbs free energy, we gain a comprehensive view of reaction spontaneity and the significance of heat exchange in chemical and physical transformations. While a negative ΔH is a strong indicator of spontaneity, the complete picture of spontaneity requires considering entropy and Gibbs free energy. The magnitude of ΔH is influenced by several factors, including bond energies, reaction stoichiometry, and the states of matter involved. Through careful analysis of ΔH values and other thermodynamic parameters, we can predict and control the outcome of numerous chemical and physical processes.
Latest Posts
Latest Posts
-
What State Of Matter Takes The Shape Of Its Container
Mar 14, 2025
-
What Is All The Factors Of 56
Mar 14, 2025
-
What Gas Is Released During Photosynthesis
Mar 14, 2025
-
Round The Number To The Nearest Thousandth
Mar 14, 2025
-
Digestion Of Starch Begins In The
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Does A Negative Delta H Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
