What Causes The Periodicity In The Periodic Table
Juapaving
Mar 29, 2025 · 7 min read
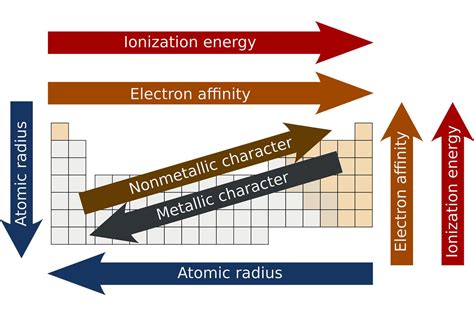
Table of Contents
What Causes the Periodicity in the Periodic Table?
The periodic table, a cornerstone of chemistry, is renowned for its inherent periodicity—the recurring trends in the physical and chemical properties of elements. This remarkable pattern isn't arbitrary; it stems directly from the quantum mechanical behavior of electrons within atoms. Understanding this connection is key to unlocking a deeper appreciation of the table's structure and the predictable properties of the elements it organizes.
The Foundation: Electron Configuration and Quantum Numbers
The periodicity we observe is a direct consequence of how electrons are arranged within an atom. This arrangement is governed by four quantum numbers:
-
Principal Quantum Number (n): This number defines the electron shell, or energy level, and dictates the electron's average distance from the nucleus. Higher 'n' values correspond to higher energy levels and greater distances.
-
Azimuthal Quantum Number (l): This describes the subshell within a shell, representing the electron's orbital angular momentum. 'l' can range from 0 to (n-1), leading to subshells denoted as s (l=0), p (l=1), d (l=2), and f (l=3). Each subshell has a characteristic shape.
-
Magnetic Quantum Number (ml): This specifies the orientation of the orbital in space. For a given 'l', 'ml' can range from -l to +l, including 0. For example, a p subshell (l=1) has three orbitals (ml = -1, 0, +1).
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum of the electron, often visualized as a spinning motion. It can only have two values: +1/2 (spin up) or -1/2 (spin down). The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers; each orbital can hold a maximum of two electrons with opposite spins.
These quantum numbers dictate the electron configuration of an atom, specifying the number of electrons in each subshell. This configuration, in turn, determines the atom's chemical and physical properties.
Building the Table: Electron Shells and Periods
The periodic table is structured in periods (rows) and groups (columns). The period number corresponds directly to the principal quantum number (n) of the outermost electron shell being filled.
-
Period 1: Contains only hydrogen (1s<sup>1</sup>) and helium (1s<sup>2</sup>), filling the n=1 shell.
-
Period 2: Elements in this period fill the n=2 shell, which includes the 2s and 2p subshells. This period contains 8 elements (Li to Ne).
-
Period 3: Similar to period 2, this period fills the n=3 shell (3s and 3p subshells), also containing 8 elements (Na to Ar).
The predictable filling of electron shells explains the length of each period. The number of elements in a period increases as we move down the table, reflecting the increasing number of subshells available at higher energy levels.
Groups and Valence Electrons: The Key to Chemical Behavior
The groups or families (columns) of the periodic table reflect the similar chemical properties of elements within each group. This similarity arises from the identical number of valence electrons, which are the electrons in the outermost shell. These valence electrons are the primary players in chemical bonding.
-
Group 1 (Alkali Metals): These elements all have one valence electron (ns<sup>1</sup> configuration), making them highly reactive and readily losing this electron to form +1 ions.
-
Group 18 (Noble Gases): These elements have a full valence shell (ns<sup>2</sup>np<sup>6</sup> configuration), making them exceptionally stable and unreactive. Their full octet (eight valence electrons) represents a state of low energy.
-
Other Groups: The number of valence electrons determines the typical oxidation states and bonding behavior of elements in other groups. For example, Group 14 elements (like carbon and silicon) have four valence electrons and can form four covalent bonds.
The repeating pattern of valence electron configurations as we move down a group explains the periodic recurrence of chemical properties. Elements in the same group exhibit similar chemical behavior because their atoms have a similar tendency to gain, lose, or share electrons to achieve a stable electron configuration.
Trends in Properties: Atomic Radius, Ionization Energy, and Electronegativity
The periodic table isn't just a list of elements; it also reveals systematic trends in various properties. These trends are directly linked to the electronic structure and effective nuclear charge.
Atomic Radius
Atomic radius generally increases down a group (due to the addition of electron shells) and decreases across a period (due to increased effective nuclear charge). The effective nuclear charge is the net positive charge experienced by valence electrons, considering the shielding effect of inner electrons. Across a period, the increased effective nuclear charge pulls the valence electrons closer to the nucleus, reducing the atomic radius.
Ionization Energy
Ionization energy is the energy required to remove an electron from a gaseous atom. It decreases down a group (due to increasing atomic radius and weaker attraction to the nucleus) and increases across a period (due to increasing effective nuclear charge and stronger attraction to the nucleus).
Electronegativity
Electronegativity is the ability of an atom to attract electrons in a chemical bond. It decreases down a group (due to increasing atomic radius and decreasing attraction to electrons) and increases across a period (due to increasing effective nuclear charge and stronger attraction to electrons).
Beyond the Basics: d-Block and f-Block Elements
The transition metals (d-block elements) and inner transition metals (f-block elements) add complexity to the periodic table. Their properties are less straightforwardly predictable due to the involvement of inner d and f orbitals. However, even within these blocks, trends and patterns exist, though they are often less pronounced than in the s- and p-blocks. The filling of these inner orbitals influences the properties of transition metals, leading to variable oxidation states and complex coordination chemistry. The lanthanides and actinides (f-block) display very similar chemical properties due to the shielding effect of the f electrons.
The Role of Quantum Mechanics: A Deeper Dive
The periodicity of the periodic table is not just a convenient observation; it is a direct consequence of the quantum mechanical model of the atom. The wave nature of electrons and the quantization of energy levels dictate the allowed electron configurations. The solutions to the Schrödinger equation for the hydrogen atom, and subsequent approximations for multi-electron atoms, underpin the entire structure.
The shapes of atomic orbitals (s, p, d, f) and their spatial orientations, arising from the solutions to the Schrödinger equation, directly influence the chemical bonding capabilities and the resulting properties. The energy levels of these orbitals determine the order of electron filling and ultimately dictate the periodicity.
In essence, the periodic table is a visual manifestation of the fundamental laws of quantum mechanics as they apply to atoms and their electron configurations. The repeating patterns arise from the regular filling of electron shells and subshells, which in turn governs the chemical behavior and physical properties of the elements. Understanding this profound connection between quantum mechanics and the periodic table is crucial for a complete grasp of chemistry and its predictive power.
Conclusion: A Powerful Organizing Principle
The periodic table's periodicity isn't merely a classification system; it's a powerful tool for understanding and predicting the properties of elements. The underlying principles—electron configuration, quantum numbers, and the resulting trends in properties—provide a framework for rationalizing the behavior of matter and developing new materials and technologies. Its elegance and predictive power underscore the fundamental importance of the quantum mechanical description of the atom. The table is not just a historical artifact; it remains a living testament to the power of scientific inquiry and the fundamental laws that govern our universe. The recurring patterns, driven by the quantum behavior of electrons, continue to guide and inspire research in chemistry and related fields.
Latest Posts
Latest Posts
-
The Pitch Of A Sound Is Determined By What
Apr 01, 2025
-
Is 2 3 Less Than 1 2
Apr 01, 2025
-
Is 23 A Prime Number Or Composite
Apr 01, 2025
-
Is The Square Root Of 45 A Rational Number
Apr 01, 2025
-
Which Phase Do Chromosomes First Become Visible
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Causes The Periodicity In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
