What Are Two Properties Of Ionic Compounds
Juapaving
Mar 22, 2025 · 6 min read
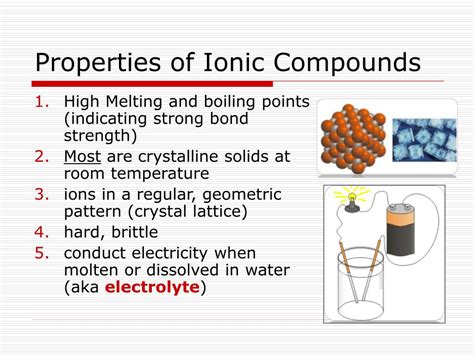
Table of Contents
What are Two Properties of Ionic Compounds? Delving Deep into Crystal Structure and Conductivity
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit a fascinating array of properties. While a comprehensive list would be extensive, two key properties stand out due to their fundamental connection to the ionic bond: high melting and boiling points, and electrical conductivity. This article will delve deeply into these properties, exploring the underlying reasons for their existence and the nuances that govern their behavior. We'll also explore how these properties relate to the broader applications of ionic compounds in various fields.
High Melting and Boiling Points: The Strength of Electrostatic Attraction
One of the most defining characteristics of ionic compounds is their remarkably high melting and boiling points. This is a direct consequence of the strong electrostatic forces holding the ions together in a rigid, crystalline lattice structure. Let's break this down:
Understanding the Crystal Lattice
Ionic compounds don't exist as individual molecules; instead, they form extensive three-dimensional crystal lattices. Imagine a highly organized array of positively charged cations and negatively charged anions, meticulously arranged to maximize electrostatic attraction and minimize repulsion. This arrangement is not random; it's dictated by the sizes and charges of the constituent ions. For example, in sodium chloride (NaCl, common table salt), sodium cations (Na⁺) and chloride anions (Cl⁻) alternate in a cubic structure.
The strength of the electrostatic attraction is directly proportional to the charges of the ions and inversely proportional to the distance between them (Coulomb's Law). Higher charges lead to stronger attractions, while larger ions, being further apart, experience weaker forces. This means that compounds with highly charged ions (e.g., MgO, with Mg²⁺ and O²⁻) have significantly higher melting and boiling points compared to those with singly charged ions (e.g., NaCl).
The Energy Barrier to Melting and Boiling
To melt an ionic compound, sufficient energy must be supplied to overcome the strong electrostatic forces holding the ions in the lattice. This energy, typically in the form of heat, must disrupt the ordered arrangement, allowing the ions to move more freely. Similarly, boiling requires even more energy to completely overcome the attractive forces and transition the compound from a liquid to a gas phase.
The high energy barrier explains why ionic compounds generally have significantly higher melting and boiling points than covalent compounds, which are held together by weaker intermolecular forces. This property has important implications in many applications, from material science to industrial processes.
Factors Affecting Melting and Boiling Points
While the strength of the ionic bond is the primary determinant, other factors also influence the melting and boiling points of ionic compounds:
- Ionic Size: Smaller ions lead to stronger electrostatic attractions and higher melting/boiling points.
- Ionic Charge: Higher ionic charges result in stronger attractions and higher melting/boiling points.
- Lattice Structure: The arrangement of ions in the crystal lattice can influence the overall strength of the structure and thus the melting/boiling point. More efficient packing leads to higher melting and boiling points.
- Polarizability: While less dominant than ionic charge and size, the polarizability of ions can influence the strength of the interactions, affecting melting and boiling points.
Understanding these factors allows for predicting and manipulating the melting and boiling points of ionic compounds, which is crucial in various applications like designing high-temperature materials.
Electrical Conductivity: Ions in Motion
Another crucial property of ionic compounds is their ability to conduct electricity, but only under specific conditions. Unlike metals, which conduct electricity in the solid state due to the free movement of electrons, ionic compounds are typically insulators in their solid state.
Conductivity in the Molten State and in Solution
The key to ionic conductivity lies in the mobility of ions. In the solid state, ions are locked in the rigid crystal lattice, restricting their movement. However, when an ionic compound is melted (fused) or dissolved in a polar solvent like water, the ions become free to move. This mobility is essential for electrical conductivity.
When an electric field is applied to molten ionic compounds or their solutions, the positively charged cations migrate towards the negative electrode (cathode), and the negatively charged anions move towards the positive electrode (anode). This movement of charge carriers constitutes an electric current. The higher the concentration of ions and their mobility, the greater the conductivity.
Electrolysis: Harnessing Ionic Conductivity
The process of using electricity to drive chemical changes in molten ionic compounds or their solutions is called electrolysis. This technique has significant industrial applications, including:
- Electrorefining of metals: Impure metals can be purified by electrolyzing their molten salts.
- Electroplating: Coating a metal object with another metal using electrolysis.
- Production of chemicals: Electrolysis is used to produce various chemicals like sodium hydroxide (NaOH), chlorine (Cl₂), and hydrogen (H₂).
Factors Affecting Conductivity
Several factors influence the electrical conductivity of ionic compounds:
- Concentration of ions: Higher ion concentration leads to higher conductivity.
- Temperature: Higher temperatures increase ion mobility, leading to higher conductivity (in molten state and solutions).
- Solvent polarity: Polar solvents effectively dissolve ionic compounds, leading to higher conductivity compared to nonpolar solvents.
- Ion size and charge: Smaller ions with higher charges generally exhibit higher mobility and thus contribute to better conductivity.
Understanding these factors is crucial for optimizing the efficiency of electrolytic processes and designing effective electrolytes for various applications.
Applications Leveraging These Properties
The high melting points and electrical conductivity of ionic compounds are exploited in numerous technological applications:
High-Temperature Applications:
- Refractory materials: Ionic compounds with exceptionally high melting points are used in high-temperature applications like furnace linings and crucibles.
- Ceramics: Many ceramics are based on ionic compounds, offering high strength and thermal resistance.
Electrolyte Applications:
- Batteries: Ionic compounds serve as electrolytes in many battery systems, enabling the flow of ions and enabling the generation of electricity. Different ionic compounds are chosen based on their conductivity, stability, and safety.
- Fuel cells: Ionic compounds are essential components of fuel cells, facilitating the transport of ions between electrodes.
- Electrolytic capacitors: These capacitors utilize the conductivity of ionic compounds in solutions for energy storage.
Other Applications:
- Medicine: Many ionic compounds play crucial roles in biological processes and are used in medications.
- Agriculture: Ionic compounds are vital components of fertilizers, providing essential nutrients to plants.
- Food industry: Table salt (NaCl) is an essential ingredient and preservative in the food industry.
Conclusion: The Significance of Ionic Properties
The high melting and boiling points and electrical conductivity of ionic compounds are not simply interesting facts; they are fundamental properties that dictate their behavior and enable their wide range of applications. Understanding the underlying reasons for these properties – the strength of electrostatic attraction and the mobility of ions – is crucial for predicting the behavior of these materials and developing new technologies. Further research continues to explore and refine our understanding of ionic compounds, leading to innovative materials and processes with far-reaching implications for diverse fields. The study of ionic compounds is a testament to the power of fundamental principles in driving technological advancements.
Latest Posts
Latest Posts
-
Are There Mitochondria In Plant Cells
Mar 23, 2025
-
Angle Of Intersection Between Two Curves
Mar 23, 2025
-
How Are Rectangles And Squares Alike
Mar 23, 2025
-
An Angle That Measures Exactly 180 Degrees
Mar 23, 2025
-
State Any Three Merits Of Democracy
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Are Two Properties Of Ionic Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
