What Are Three Examples Of Chemical Changes
Juapaving
Mar 31, 2025 · 6 min read
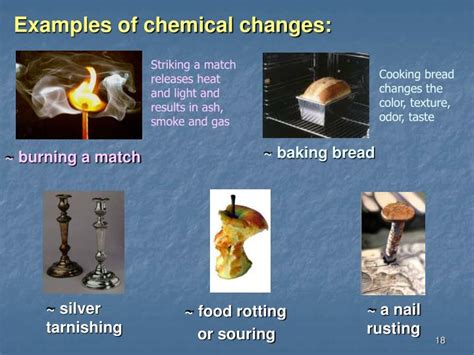
Table of Contents
What Are Three Examples of Chemical Changes? Exploring Transformations in Matter
Understanding chemical changes is fundamental to grasping the world around us. Unlike physical changes, which alter the form but not the composition of a substance (like melting ice), chemical changes involve a rearrangement of atoms and molecules, resulting in entirely new substances with different properties. This article will delve into three compelling examples of chemical changes, illustrating the key characteristics that distinguish them from their physical counterparts. We will explore the underlying chemistry, observe the observable evidence, and discuss the practical applications of these transformations.
Example 1: Combustion – The Fiery Transformation
Combustion, or burning, is perhaps the most readily recognizable chemical change. It's a rapid reaction between a fuel and an oxidant (usually oxygen) that produces heat and light. This process fundamentally alters the chemical composition of the fuel, creating new products, often with significantly different properties.
The Chemistry of Combustion
Let's consider the classic example: burning wood. Wood is primarily composed of cellulose, a complex carbohydrate. When you set wood alight, the cellulose reacts with oxygen in the air. This reaction breaks down the cellulose molecules into simpler molecules like carbon dioxide (CO2), water (H2O), and various other gases and ash. The chemical equation for the simplified combustion of cellulose can be represented as:
(C6H10O5)n + 6nO2 → 6nCO2 + 5nH2O + Energy
Where 'n' represents the number of glucose units in the cellulose molecule.
This equation showcases the core principle of combustion: the rearrangement of atoms from the reactants (cellulose and oxygen) to form entirely new products (carbon dioxide and water). The energy released as heat and light is another crucial indicator of a chemical change.
Observable Evidence of Combustion
Several easily observable characteristics confirm that combustion is a chemical change:
- Production of Heat and Light: The most obvious indicator is the generation of flames and intense heat. This energy release stems from the breaking and forming of chemical bonds during the reaction.
- Formation of New Substances: The original wood is transformed into ash, carbon dioxide, and water vapor. These substances have completely different properties from the starting material. The ash is a residue of inorganic minerals present in the wood, while the CO2 and H2O are gases that dissipate into the atmosphere.
- Irreversibility: You cannot easily reverse the process of burning wood to regain the original wood. This irreversibility is a hallmark of most chemical changes.
Applications of Combustion
Combustion plays a vital role in various aspects of our lives:
- Energy Production: Combustion fuels power our cars, generate electricity in power plants, and heat our homes. The controlled burning of fossil fuels (coal, oil, and natural gas) remains a major source of energy worldwide.
- Industrial Processes: Combustion is used in various industrial processes such as metal refining, cement production, and waste incineration.
- Cooking: Cooking utilizes combustion to produce heat for preparing food.
Example 2: Rusting – The Slow Oxidation of Iron
Rusting, also known as corrosion, is a slower chemical change compared to combustion. It's an oxidation-reduction reaction where iron reacts with oxygen and water to form iron oxide, commonly known as rust. This process gradually weakens the iron structure, eventually leading to its deterioration.
The Chemistry of Rusting
The rusting process involves a complex series of reactions, but the simplified equation can be represented as:
4Fe(s) + 3O2(g) + 6H2O(l) → 4Fe(OH)3(s)
Where:
- Fe(s) represents solid iron.
- O2(g) represents oxygen gas.
- H2O(l) represents liquid water.
- Fe(OH)3(s) represents iron(III) hydroxide, a component of rust.
This reaction shows how iron atoms combine with oxygen and water molecules to create a new compound – iron(III) hydroxide – which is the primary constituent of rust. This transformation is irreversible, and the resulting rust has vastly different properties than the original iron.
Observable Evidence of Rusting
Several signs indicate that rusting is a chemical change:
- Color Change: The characteristic reddish-brown color of rust is a clear indication of a chemical transformation. Iron's metallic gray color is replaced by the distinctive rusty hue.
- Formation of a New Substance: The formation of a flaky, brittle substance (rust) on the surface of the iron is evidence of a new compound being formed. This rust is significantly less strong and durable than the original iron.
- Irreversibility: Removing rust from iron does not restore the original iron; it simply removes the product of the chemical change.
Applications (or rather, mitigations) of Rusting
While rusting is detrimental in many applications, understanding the process allows us to mitigate its effects:
- Protective Coatings: Painting, galvanizing (coating with zinc), and other protective coatings prevent iron from coming into contact with oxygen and water, thus inhibiting rust formation.
- Alloying: Creating alloys of iron, such as stainless steel, increases resistance to corrosion.
- Corrosion Inhibitors: Chemical substances can be added to prevent or slow down the rusting process.
Example 3: Photosynthesis – The Green Chemical Reaction
Photosynthesis is a remarkable chemical change performed by plants and other photosynthetic organisms. It's the process by which they convert light energy into chemical energy in the form of glucose. This fundamental process sustains most life on Earth.
The Chemistry of Photosynthesis
The overall equation for photosynthesis is:
6CO2 + 6H2O + Light Energy → C6H12O6 + 6O2
Where:
- CO2 represents carbon dioxide.
- H2O represents water.
- C6H12O6 represents glucose (a sugar).
- O2 represents oxygen.
Photosynthesis involves a complex series of reactions, but this simplified equation shows the transformation of carbon dioxide and water into glucose and oxygen using light energy. The glucose serves as the plant's food source, providing the energy for growth and other metabolic processes.
Observable Evidence of Photosynthesis
Several observations confirm photosynthesis as a chemical change:
- Production of Oxygen: Plants release oxygen as a byproduct of photosynthesis. This oxygen is crucial for the respiration of most living organisms.
- Production of Glucose: The plant uses the produced glucose for its growth and development, evident in the increased biomass of the plant over time.
- Color Change (in some cases): Some plants exhibit changes in leaf color due to the pigments involved in photosynthesis. This change isn't always directly observable, but it demonstrates alterations within the plant's chemical composition.
Applications of Photosynthesis (and its importance)
Photosynthesis is the cornerstone of life on Earth:
- Oxygen Production: It is the primary source of atmospheric oxygen, essential for the survival of most aerobic organisms.
- Food Production: Photosynthesis forms the basis of the food chain, providing energy for all living organisms, directly or indirectly.
- Biomass Production: Plants, through photosynthesis, provide biomass used for various purposes like fuel, building materials, and textiles.
- Carbon Sequestration: Photosynthesis absorbs carbon dioxide from the atmosphere, playing a critical role in regulating Earth's climate.
Conclusion: Recognizing Chemical Changes
These three examples – combustion, rusting, and photosynthesis – illustrate the diverse nature of chemical changes. They demonstrate that chemical changes involve the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Recognizing the characteristic signs of these transformations – such as heat and light production, color changes, gas evolution, and the formation of precipitates – is crucial for understanding the chemical processes that shape our world. Understanding these fundamental changes underpins advancements in various fields, from energy production and materials science to agriculture and environmental science. By grasping the principles behind these chemical reactions, we can better appreciate the dynamic nature of matter and the intricate processes that sustain life on Earth.
Latest Posts
Latest Posts
-
Moment Of Inertia Of A Circle Formula
Apr 01, 2025
-
A Solenoid With A Core Is Called An Electromagnet
Apr 01, 2025
-
In An Exponential Function What Does The A Represent
Apr 01, 2025
-
When Is A Substance A Limiting Nutrient
Apr 01, 2025
-
Beryllium Atomic Number And Mass Number
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Are Three Examples Of Chemical Changes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
