What Are The Three Basic Parts Of A Nucleotide
Juapaving
Mar 24, 2025 · 5 min read
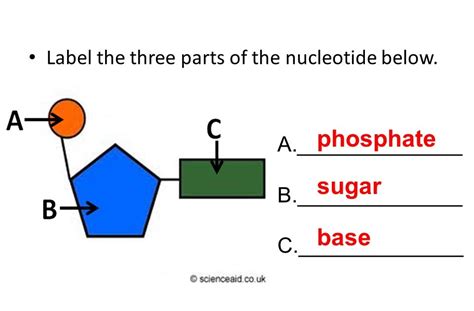
Table of Contents
What are the Three Basic Parts of a Nucleotide? A Deep Dive into the Building Blocks of Life
Nucleotides, the fundamental building blocks of nucleic acids like DNA and RNA, are complex molecules with a surprisingly simple core structure. Understanding their components is crucial to grasping the intricacies of genetic information storage, transfer, and expression. This article will delve into the three basic parts of a nucleotide, exploring their chemical properties, roles within the molecule, and significance in biological processes. We'll also touch upon the variations seen in nucleotides and their broader implications in the field of molecular biology.
The Tripartite Structure: Sugar, Base, and Phosphate
A nucleotide is composed of three essential components: a pentose sugar, a nitrogenous base, and a phosphate group. These components are linked together in a specific manner to create the nucleotide monomer, which then polymerizes to form the polynucleotide chains of DNA and RNA.
1. The Pentose Sugar: The Sweet Backbone
The pentose sugar is a five-carbon sugar molecule that forms the structural backbone of the nucleotide. There are two main types of pentose sugars found in nucleotides:
- Ribose: Found in ribonucleotides, the building blocks of RNA. Ribose has a hydroxyl (-OH) group attached to the 2' carbon atom.
- Deoxyribose: Found in deoxyribonucleotides, the building blocks of DNA. Deoxyribose lacks the hydroxyl group at the 2' carbon atom, hence the "deoxy" prefix. This seemingly small difference has profound implications for the stability and function of DNA compared to RNA.
The numbering of carbon atoms in the pentose sugar is crucial for understanding the linkage between the different components of the nucleotide. The carbon atoms are numbered 1' through 5', with the prime symbol distinguishing them from the atoms in the nitrogenous base. The hydroxyl groups on the 3' and 5' carbons are particularly important because they are involved in the formation of phosphodiester bonds that link nucleotides together to form the polynucleotide chain.
Why is the difference between ribose and deoxyribose significant? The presence of the 2'-hydroxyl group in ribose makes RNA more susceptible to hydrolysis (breaking down in the presence of water) compared to DNA. This inherent instability contributes to RNA's typically shorter lifespan and its role in transient processes compared to DNA's long-term storage of genetic information.
2. The Nitrogenous Base: The Information Carrier
The nitrogenous base is a crucial component of the nucleotide, carrying the genetic information. These bases are heterocyclic organic molecules containing nitrogen atoms. They are classified into two main groups:
- Purines: These are larger, double-ringed structures. Adenine (A) and guanine (G) are purine bases found in both DNA and RNA.
- Pyrimidines: These are smaller, single-ringed structures. Cytosine (C) is found in both DNA and RNA, while thymine (T) is found exclusively in DNA, and uracil (U) is found exclusively in RNA.
The nitrogenous base is attached to the 1' carbon of the pentose sugar via a glycosidic bond. This bond forms the nucleoside. The specific nitrogenous base attached to the sugar determines the nucleotide's identity (e.g., adenosine monophosphate, guanosine monophosphate, etc.). The sequence of these bases along the polynucleotide chain determines the genetic code.
Base Pairing: The specific pairing of nitrogenous bases is a cornerstone of DNA structure and function. Adenine (A) always pairs with thymine (T) in DNA (or uracil (U) in RNA) through two hydrogen bonds, while guanine (G) always pairs with cytosine (C) through three hydrogen bonds. This complementary base pairing is essential for DNA replication and transcription.
3. The Phosphate Group: The Energetic Link
The phosphate group is a negatively charged molecule consisting of a phosphorus atom bonded to four oxygen atoms. It is typically attached to the 5' carbon of the pentose sugar through a phosphoester bond. The phosphate group plays several vital roles:
- Linking Nucleotides: The phosphate group acts as a bridge, linking the 5' carbon of one nucleotide to the 3' carbon of the next nucleotide through a phosphodiester bond. This forms the sugar-phosphate backbone of the polynucleotide chain, providing structural integrity and directionality (5' to 3').
- Energy Carrier: Nucleotides containing multiple phosphate groups, such as adenosine triphosphate (ATP), are crucial energy carriers in cells. The hydrolysis of these high-energy phosphate bonds releases energy that fuels various cellular processes.
- Regulatory Roles: Nucleotides also participate in signal transduction pathways and act as regulators of various enzymatic reactions.
Number of Phosphate Groups: Nucleotides can exist with varying numbers of phosphate groups attached. A nucleoside monophosphate (NMP) has one phosphate group, a nucleoside diphosphate (NDP) has two, and a nucleoside triphosphate (NTP) has three. NTPs are particularly important as they serve as the precursors for DNA and RNA synthesis. The high-energy bonds in NTPs provide the energy needed for the polymerization reaction.
Variations and Significance
The basic structure of a nucleotide is remarkably consistent across all life forms. However, there are variations that exist, particularly in the modified bases found in certain RNA molecules and in the presence of unusual sugars or phosphate analogs.
Modified Bases: Many RNA molecules contain modified bases, such as pseudouridine, inosine, and methylated bases. These modifications often play critical roles in RNA structure, stability, and function. For instance, they can influence RNA folding, binding to proteins, and interactions with other RNA molecules.
Unusual Sugars and Phosphate Analogs: Synthetic nucleotides incorporating modified sugars or phosphate analogs are valuable tools in molecular biology research. These analogs can be used to study nucleotide metabolism, to design new therapeutic agents, or to modify the properties of nucleic acids for specific applications.
Conclusion: A Building Block with Far-Reaching Implications
The seemingly simple structure of a nucleotide—a pentose sugar, a nitrogenous base, and a phosphate group—underlies the incredible complexity of life. Understanding the individual components and their interactions is essential for comprehending the mechanisms of DNA replication, transcription, translation, and numerous other vital biological processes. The variations in nucleotide structure and function also highlight the adaptability and sophistication of biological systems, opening up exciting avenues for research and technological advancements. From the energetic role of ATP to the information storage capacity of DNA, the nucleotide remains a central molecule in the study of life itself.
Latest Posts
Latest Posts
-
Difference Between Composite Materials And Alloys
Mar 28, 2025
-
How Many Valence Electrons Are In Oxygen
Mar 28, 2025
-
Which Of The Following Letters Does Not Suffer Lateral Inversion
Mar 28, 2025
-
Is 53 A Prime Number Or A Composite Number
Mar 28, 2025
-
What Is The Least Common Multiple Of 24 And 15
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Are The Three Basic Parts Of A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
